Hexabromocyclododecane
- CAS NO.:25637-99-4
- Empirical Formula: C12H18Br6
- Molecular Weight: 641.7
- MDL number: MFCD00003716
- EINECS: 247-148-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-01 18:33:23
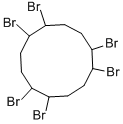
What is Hexabromocyclododecane?
Description
Hexabromocyclododecane (HBCD) is a bromine-containing flame retardant used primarily in polystyrene insulating foam. It is used in very low amounts (0.2–0.7% in the foam), but it accumulates in the environment and is toxic to aquatic organisms and laboratory animals. Earlier this year, member countries of the Stockholm Convention on Persistent Organic Pollutants?agreed to end HBCD production, but a ban on its use will not go into effect until 2019. Current HBCD producers have committed to developing alternative flame retardants.
HBCD is a mixture of 16 diastereomers. One of the more abundant ones, (–)-α, is shown in the figure.
Chemical properties
Hexabromocyclododecane (HBCD) is a white odorless nonvolatile solid compound which is used as an additive flame retardant on its own, or in combination with other flame retardants. It is used as a flame retardant mainly in building insulation composed of extruded or expanded polystyrene foam. A minor use is in flame retardant back-coats of some upholstery textiles.
The Uses of Hexabromocyclododecane
The commercially available brominated flame retardant hexabromocyclododecane (HBCD) is lipophilic, has a high affinity to particulate matter and low water solubility. It is used as a flame retardant additive, with the intent of delaying ignition and slowing subsequent fire growth during the service life of vehicles, buildings or articles, as well as while materials are stored. The main uses of HBCD are in flame-retarded expanded (EPS) and extruded (XPS) polystyrene foam insulation, with smaller scale use in textile applications and electric and electronic appliances (high impact polystyrene/HIPS). In textiles HBCD is used in back-coatings for upholstery and other interior textiles, including automotive applications.
Preparation
The aliphatic flame retardant, hexabromocyclododecane (HBCD), is produced by the addition reaction of bromine to trans,trans,cis-1,5,9-cyclododecatriene, the cyclic trimer of butadiene. The HBCD that is obtained from this reaction consists of a mixture of stereoisomers of 1,2,5,6,9,10-hexabromocyclo-dodecane [3194-55-6] ranging from oils to an isomer with a 205–208℃ melting point. Commercially available HBCD contains a mixture of solid isomers and has a melting point range of 170–180 ℃ (185–195℃ ).
Addition of bromine to the cyclic triene in nonpolar solvents results in substantial amounts of free-radical substitution reactions which lead to decreased yields and lower quality product. Use of polar solvents, such as ethanol, reduces the substitution reaction but can give rise to vicinal bromoethoxy by products. Mixed solvent systems, such as benzene–tert-butyl alcohol or perchloroethylene-ethanol,can be employed to improve product purities and yields.
Another technique to reduce the formation of undesired byproducts is to add the bromine and cyclododecatriene simultaneously and sep- arately to ethanol. This process is carried out by separately feeding bromine and cyclo- dodecatriene in a molar ratio of 3 : 1 to 96 % ethanolinastirredreactor.The reaction temperature is kept at 20-30℃ and a 1-5% excess of bromine is maintained during the 2.5–3.0h addition. The precipitated product is recovered by filtration or centrifugation, and the mother liquor is recovered and reused in subsequent reactions after adding makeup ethanol. The total yield of dried HBCD from nine separate batches using recycled mother liquor is 92%.
General Description
HexabroMocyclododecane is White powder. Insoluble in water.
Air & Water Reactions
HexabroMocyclododecane is Insoluble in water.
Reactivity Profile
Halogenated aliphatic compounds, such as Hexabromocyclododecane, are moderately or very reactive. Reactivity generally decreases with increased degree of substitution of halogen for hydrogen atoms. Materials in this group may be incompatible with strong oxidizing and reducing agents. Also, they may be incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. Hexabromocyclododecane can react vigorously with oxidizing materials.
Fire Hazard
Flash point data for Hexabromocyclododecane are not available. Hexabromocyclododecane is probably combustible.
Properties of Hexabromocyclododecane
| Melting point: | 188-191 °C |
| Density | 1.05 g/cm3 |
| vapor pressure | 0Pa at 21℃ |
| solubility | Benzene (Slightly), Ethyl Acetate (Slightly) |
| form | Solid |
| color | White to Off-White |
| Water Solubility | 65.6μg/L at 20℃ |
| CAS DataBase Reference | 25637-99-4(CAS DataBase Reference) |
| EPA Substance Registry System | Hexabromocyclododecane (25637-99-4) |
Safety information for Hexabromocyclododecane
Computed Descriptors for Hexabromocyclododecane
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
