Hesperidin
Synonym(s):3′,5,7-Trihydroxy 4′-methoxyflavanone 7-rutinoside;Hesperetin 7-rhamnoglucoside;Hesperidin;Hesperitin-7-rutinoside
- CAS NO.:520-26-3
- Empirical Formula: C28H34O15
- Molecular Weight: 610.56
- MDL number: MFCD00075663
- EINECS: 208-288-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-25 11:31:46
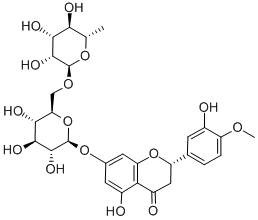
What is Hesperidin?
Description
Hesperidin exists mainly in the peel of lemon, orange, and Seville orange flower,
which belong to facilitating medicine. These Chinese medicines are warm and fragrant with the function of eliminating depression and knots. They cure abdominal
distension, belching swallow acid pain, nausea and vomiting, diarrhea or constipation caused by the spleen, and stomach qi zhi; and they also cure depression, hernia,
breast pain, and menoxenia caused by liver qi; moreover, they cure chest pain,
cough, and asthma, which are caused by lung qi.
Modern research has shown that li qi medicines have an extensive effect, such as
regulatory effect on the digestive system, and they control the bronchial smooth
muscle, the uterus smooth muscle, and the cardiovascular system. The base of the
effect of li qi medicines in inverse, anti-nausea, antidiarrheal, and analgesia pharmacological effects is its effect in inhibiting gastric bowel movement; its exciting gastrointestinal movement is the foundation to eliminate swelling; its effect in relaxation
of the bronchial smooth muscle is the foundation of pharmacological effects in antinausea. The intravenous injection to treat shock effect is the new development of li
qi medicine.
Description
Hesperidin is a flavanone rutinoside first isolated from citrus peels. It is metabolized by intestinal bacteria to an aglycone form, hesperetin (Item No. 10006084), which is thought to be more bioavailable due to reduced polarity that allows for increased cell permeability. At 100 μM, hesperidin has been shown to increase the cytotoxicity of doxorubicin on MCF-7 and HeLa cancer cells in vitro by inhibiting cell cycle progression and upregulating apoptosis. It also is reported to produce estrogenic effects, decreasing serum and hepatic lipid concentrations and reducing osteoporosis in ovariectomized rats. Hesperidin produces free radical scavenging activity in various in vitro antioxidant assays.
Chemical properties
light brown powder
Physical properties
Appearance: fine dendritic crystal (precipitation at pH 6–7), odorless, tasteless. Melting point: 258–262?°C (softening at 250?°C). Solubility: 1?g of hesperidin can be soluble in 50?l of water. Hesperidin dissolves in dimethyl formamide and formamide at 60?°C.?It is slightly soluble in methanol and hot ice acetic acid and hardly soluble in acetone, benzene, and chloroform and is soluble in dilute alkali and pyridine.
History
Hesperidin is the glycoside in the form of hesperidin and rubiose and is a derivative of dihydroflavonoids. It widely exists in legume, birch, lip flower, butterfly flower, Rutaceae, and citrus plants. Hesperidin is an important composition of citrus pulp and peel; most of hesperidin exists in citrus processing waste such as skin and fruit bag. Mature skin and tissue have the highest content of hesperidin (30–50% in endocarp; 30–50% in orange collaterals, nuclear, and pulp; and 10–20% in exo_x005fcarp); the content of hesperidin is relatively low in juice and orange bag, which is about 1–5%. The crude extracts of hesperidin was first discovered in 1827 by Lebreton. Then the Hungarian scholar Albert Szent-Gyorgi discovered that the flavonoids have a protective microvascular effect in 1936, which is similar to that of vitamin P. Preparation of vitamin P was made in 1938. It was not until 1949 that it was discovered that vitamin P was made up of two flavonoids, luteolin and hesperidin, which are believed to be vitamin active. This substance, which was later named as vitamin P, was designed to reduce blood vessel permeability and brittleness, as well as alleviate bad blood and vitamin C deficiency. It was later discovered that the substance had an antioxidant effect, so the name of vitamin P was abandoned. Due to the widespread distribution of hesperidin in plant medicine, the research and development have been widely followed.
The Uses of Hesperidin
Hesperidin is a flavoring agent that is a bioflavonoid found in citrus pulp. it has minor use as a flavorant.
The Uses of Hesperidin
A flavanone found in citrus fruits, also regarded as Vitamin P.
The Uses of Hesperidin
antiinflammatory, capillary protectant, hypolipidemic
The Uses of Hesperidin
Anticancer
The Uses of Hesperidin
vitamin P is considered a vascular protector and an anti-inflammatory agent, vitamin P is said to promote capillary health and increase resistance to collagen destruction. Vitamin P is a bioflavonoid that can work in conjunction with vitamin C, helping prevent oxidation of the latter. Vitamin P is found in such food sources as apricots, broccoli, citrus fruit pulp, grapes, prunes, and spinach.
Background
Hesperidin is a flavan-on glycoside found in citrus fruits.
What are the applications of Application
Hesperidin is a chemopreventive flavonoid
Definition
ChEBI: A disaccharide derivative that consists of hesperetin substituted by a 6-O-(alpha-L-rhamnopyranosyl)-beta-D-glucopyranosyl moiety at position 7 via a glycosidic linkage.
Indications
Hesperidin can be used for cardiovascular disease prevention and treatment, blood sugar and blood lipid and blood pressure regulation, circulatory system regulation, and body regulation, and it can also be used as an antibacterial, anti-inflammatory, and antiviral.
Flammability and Explosibility
Non flammable
Pharmacology
The pharmacological effect of hesperidin is widespread, and people thought it was
vitamin P in the early days, but in recent years, people found that it has other functions such as controlling blood pressure, antiallergic, reducing bone mineral density
and cholesterol, improving enzyme activity and microcirculation, antibacterial,
anti-inflammatory, anti-hepatitis B, antitumor, and other pharmacological effects.Hesperidin has the function of vitamin P, which can reduce capillary permeability and prevent microvascular hemorrhage. Intraperitoneal injection of hesperidin at
175–250?mg/kg in mice could increase permeability of blood vessels by antihistamine and inhibiting hemolytic lecithin. Hesperidin has antiviral and antimicrobial
effect, and preincubation with hesperidin at 200? mg/ml protects the cells from
viruses. One to 10?μg/ml of hesperidin effectively inhibits the growth of the fungus.
It has the effect of maintaining the normal osmotic pressure of the blood vessels,
reducing the shortness of blood vessels, shortening bleeding time, reducing blood
fat, and preventing atherosclerosis; hesperidin has an effect on the gastrointestinal
tract, which can excite the smooth muscle transiently and then inhibit it, and it is a
major component of the diet drug; hesperidin has an effect of anti-lipid peroxidation
and scavenging hydroxyl radical. Hesperidin is a newly discovered flavonoid compound which has an effect in the central nervous system; it has a sedative effect. At
the same time, hesperidin has the effect of lowering cholesterol, curing rheumatism,
and inhibiting skin pigmentation. Hesperidin is a strong affinity for estrogen receptors, which can be used in estrogen receptors to prevent bone loss and reduce the
number of osteoblasts.
Hesperidin has a significant inhibitory effect on human lung
cancer, colorectal cancer, kidney cancer, and human breast cancer cells, which can
be used for cancer prevention.
Clinical Use
Hesperidin has the effect to maintain osmotic pressure, strengthen the capillary toughness, shorten the bleeding time, lower cholesterol, and so on. Although hesperidin cannot be used as independent medication, it is recorded in the pharmacopoeia that hesperidin, as auxiliary materials, is widely used to aid in the treatment of cardiovascular system; it can be configured as a variety of drugs to prevent hardening of the arteries and myocardial infarction. It is one of the main raw materials of medicine “pulse.” Hesperidin is used as auxiliary materials for the treatment of vascular brittleness, bedsore, rheumatoid arthritis, vitamin C deficiency disease, trauma, obstetric disease, gum inflammation, edema, and gastrointestinal tract disease in the world. Hesperidin can be used to produce an anticancer drug called diosmin. Natural antioxidant is available in the food industry. It is also used in the cosmetics industry.
Side Effects
Potential side effects of hesperidin include: abdominal pain, diarrhoea, nausea, contact dermatitis (itchy rash caused by direct contact with the substance). In a study evaluating the effects of hesperidin supplementation on heart attacks, no serious adverse reactions were reported.
Metabolism
Not Available
Purification Methods
Dissolve hesperidine in dilute aqueous alkali and precipitate it by adjusting the pH to 6-7. [Beilstein 18 III/IV 3219, 18/5 V 218.]
Properties of Hesperidin
| Melting point: | 250-255 °C (dec.)(lit.) |
| Boiling point: | 576.16°C (rough estimate) |
| alpha | -76 º (c=2,pyridine) |
| Density | 1.3290 (rough estimate) |
| refractive index | 1.5940 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Pyridine (Slightly, Sonicated) |
| form | Powder |
| pka | 7.15±0.40(Predicted) |
| color | light brown |
| Water Solubility | Insoluble in water. Soluble in organic solvents such as DMSO. |
| Sensitive | Hygroscopic |
| Merck | 14,4671 |
| BRN | 75140 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 520-26-3(CAS DataBase Reference) |
| EPA Substance Registry System | Hesperidin (520-26-3) |
Safety information for Hesperidin
Computed Descriptors for Hesperidin
| InChIKey | QUQPHWDTPGMPEX-QJBIFVCTSA-N |
Hesperidin manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 520-26-3 98%View Details
520-26-3 98%View Details
520-26-3 -
 520-26-3 Hesperidin, 95% 99%View Details
520-26-3 Hesperidin, 95% 99%View Details
520-26-3 -
 Hesperidin 97% CAS 520-26-3View Details
Hesperidin 97% CAS 520-26-3View Details
520-26-3 -
 Hesperidin CAS 520-26-3View Details
Hesperidin CAS 520-26-3View Details
520-26-3 -
 Hesperidin CAS 520-26-3View Details
Hesperidin CAS 520-26-3View Details
520-26-3 -
 Hesperidin CAS 520-26-3View Details
Hesperidin CAS 520-26-3View Details
520-26-3 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
