D(-)-Fructose
Synonym(s):D(-)-Fructose;Fruit sugar;Laevulose, Levulose;Laevulosum (Fructosum);D- (?)-Fructose
- CAS NO.:57-48-7
- Empirical Formula: C6H12O6
- Molecular Weight: 180.16
- MDL number: MFCD00148910
- EINECS: 200-333-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 14:18:24
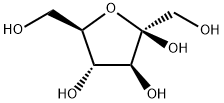
What is D(-)-Fructose?
Description
Fructose, or “fruit sugar”, is one of the three most common natural monosaccharides. (The other two are glucose and galactose.) As its name implies, fructose is found in almost all fruits; but it also exists in commercial quantities in sugarcane, sugarbeets, and corn. Fructose and glucose combine to form the disaccharide sucrose, which we know as common sugar.
The structure of fructose, like all simple sugars, can be expressed as a six-carbon linear chain with hydroxyl and carbonyl groups. In its crystalline form and in solution, however, most of it exists as two hemiketal rings: β-D-fructopyranose1 (left) and β-D-fructofuranose1?(right). In aqueous solution, it consists of 70% pyranose, 22% furanose, and smaller amounts of the linear and other cyclic forms.
Fructose is the most water-soluble monosaccharide. As indicated in the “Fast Facts” table, it dissolves in exceedingly small amounts of water. This property makes it difficult to crystallize from water and accounts for its hygroscopicity and humectancy.
Fructose was discovered by French chemist Augustin-Pierre Dubrunfaut in 1847, but the pioneering research on it and all sugars known at the time was conducted from 1884 to 1894 by German Nobel laureate Emil Fischer. Among other accomplishments, Fischer demonstrated the relationship of glucose, fructose, and mannose;and he elucidated the stereochemistry of those sugars.
Consumption of excessive quantities of foods that contain fructose and other sugars is a well-known cause of type 2 diabetes, elevated levels of LDL (“bad”) cholesterol and triglycerides, and of course, obesity. But fructose may be slightly safer than the others, especially for diabetics, because it has a lower glycemic index than sucrose and is considerably sweeter.
In 2016, Xia Yang, Fernando Gomez-Pinilla, and colleagues at UCLA and other institutions discovered that?in lab rats fed diets high in fructose, almost 1000 genes in the brains were adversely affected. In particular, fructose impaired two key genes that regulate intercellular communication.
But Yang and Gomez-Pinilla also had good news. When they fed rats docosahexaenoic acid, a key ω-3 fatty acid, along with high amounts of fructose, they saw no more gene damage than in a control group. The authors identify their study as an example of a technique called nutrigenomics, which examines the genomic bases of nutrient–host interactions that underlie disease predisposition.
1. D denotes the absolute configuration of the linear sugar; β is the more common of two ways that sugars cyclize.
Chemical properties
White Cyrstalline Solid
Chemical properties
Fructose occurs as odorless, colorless crystals or a white crystalline powder with a very sweet taste.
Originator
Levugen,Baxter,US,1953
History
Despite this ubiquity, fructose remained a noncommercial product until the 1980s because of the expense involved in its isolation and the care required for its handling. The development of technologies for preparing fructose from glucose in the isomerized mixture led to a greater availability of pure, crystalline fructose in the 1970s. However, the price for pure fructose was high enough in 1981 that the product was not competitive with sucrose and corn syrups as a commercial sweetener. With the entry of corn wet-milling companies into the crystalline fructose market in the late 1980s, raw material economies and enlarged manufacturing scale led to a nearly 10-fold production increase within a five-year period, making fructose prices competitive with other sweeteners for specific applications.
The Uses of D(-)-Fructose
D-Fructose occurs in a large number of fruits, honey, and as the sole sugar in bull and human semen
The Uses of D(-)-Fructose
fructose is a naturally occurring sugar in fruits and honey. It has moisture-binding and skin-softening properties.
The Uses of D(-)-Fructose
Fructose is a sweetener that is a monosaccharide found naturally in fresh fruit and honey. It is obtained by the inversion of sucrose by means of the enzyme invertase and by the isomerization of corn syrup. It is 130–180 in sweetness range as compared to sucrose at 100 and is very water soluble. It is used in baked goods because it reacts with amino acids to produce a browning reaction. It is used as a nutritive sweetener in low-calorie beverages. It is also termed levulose and fruit sugar.
What are the applications of Application
D-(?)-Fructose is a monosaccharide and isomer of glucose
Production Methods
Fructose, a monosaccharide sugar, occurs naturally in honey and a large number of fruits. It may be prepared from inulin, dextrose, or sucrose by a number of methods. Commercially, fructose is mainly manufactured by crystallization from high-fructose syrup derived from hydrolyzed and isomerized cereal starch or cane and beet sugar.
Definition
A sugar found in fruit juices, honey, and cane sugar. It is a ketohexose, existing in a pyranose form when free. In combination (e.g. in sucrose) it exists in the furanose form.
Manufacturing Process
200 gal of medium containing 2% sucrose, 2% corn steep liquor solids, 0.1%
potassium dihydrogen phosphate, and traces of mineral salts, was inoculated
with Leuconostoc mesenteroides NRRL B-512 and incubated at 25°C. During
growth, alkali was added automatically as needed to maintain the pH between
6.6 and 7.0. Fermentation was completed in 11 hours and the culture was
immediately adjusted to pH 5 to maintain enzyme stability. Bacterial cells
were removed by filtration and yielded a culture filtrate containing 40
dextransucrase units per ml, where one unit is the amount of dextransucrase
which will convert 1 mg of sucrose to dextran, as determined by the amount
of fructose liberated, measured as reducing power in 1 hour.
10 gal of the above culture filtrate was diluted to 40 gal with water, 33.3 lb of
sucrose was added to give a 10% solution, and toluene was added as a
preservative. Dextran synthesis was complete before 22 hours, and dextran
was harvested at 24 hours by the addition of alcohol to be 40% on a volume
basis.
The alcoholic supernatant liquor obtained was evaporated to recover the
alcohol and yielded a thick syrup, rich in fructose. Analysis showed the syrup
to contain 50.1% of reducing sugar, calculated as monosaccharide and to have
an optical rotation equivalent to 35.1% fructose. The percentages are
expressed on a weight/volume basis, and reducing power was determined by
the method of Somogyi, Jour. Biol. Chem. 160, 61 (1945). A portion (4.3
liters) of the syrup was cooled to 3°C. One-tenth of this volume was treated
by slow regular addition, with rapid stirring, of a 6-fold volume of cold 20%
calcium oxide suspension. A second portion was treated in the same manner,
and this process was continued until the entire volume of crude fructose syrup
had been utilized. The reaction mixture became thick with a white sediment
containing a profusion of microscopic needlelike crystals of calcium levulate.
Stirring was continued for 2 hours.
The calcium levulate precipitate was separated from the reaction mixture by
filtration and washed with cold water. The precipitate was suspended in water
to give a thick slurry, and solid carbon dioxide added until the solution was
colorless to phenolphthalein. A heavy precipitate of calcium carbonate was
now present and free fructose remained in the solution. The calcium
carbonate precipitate was removed by filtration, and the filtered solution was
found to contain 1,436 g of fructose as determined by optical rotation. A small
amount of calcium bicarbonate was present as an impurity in solution and was
removed by the addition of oxalic acid solution until a test for both calcium
and oxalic acid was negative. The insoluble calcium oxalate precipitate was
removed by filtration.
The fructose solution was decolorized by treatment with activated charcoal
and concentrated under vacuum to a thick syrup. Two volumes of hot 95%
ethyl alcohol were added, and the solution was heated to a boil and filtered to remove a small amount of insoluble material. After cooling, three volumes of
ethyl ether were added, and the solution was allowed to stand overnight in
the refrigerator. Fructose separated from the solution as a thick syrup and was
separated from the supernatant liquid by decantation. The syrup was seeded
with fructose crystals and after standing in the cold for 4 days, became a
crystalline mass of fructose. The yield of dry fructose was 928 g. Additional
recoverable quantities of fructose are present in the crystallization mother
liquor. In continuous operation this mother liquor may be recycled for addition
to subsequent quantities of fructose syrup and the combined liquors
crystallized as in the foregoing example.
Therapeutic Function
Fluid replenisher, Pharmaceutic aid
General Description
Fructose is a monosaccharide. It is present in fruits and vegetables. Fructose is the major carbohydrate in the diet. It binds with glucose to form sucrose. Excessive intake of fructose is associated with obesity, type 2 diabetes and cardiovascular disease.
Pharmaceutical Applications
Fructose is used in tablets, syrups, and solutions as a flavoring and
sweetening agent.
The sweetness-response profile of fructose is perceived in the
mouth more rapidly than that of sucrose and dextrose, which may
account for the ability of fructose to enhance syrup or tablet fruit
flavors and mask certain unpleasant vitamin or mineral ‘off-flavors’.
The increased solubility of fructose in comparison to sucrose is
advantageous in syrup or solution formulations that must be
refrigerated, since settling or crystallization of ingredients is
retarded. Similarly, the greater solubility and hygroscopicity of
fructose over sucrose and dextrose helps to avoid ‘cap-locking’
(sugar crystallization around the bottle cap) in elixir preparations.
Fructose also has greater solubility in ethanol (95%) and is
therefore used to sweeten alcoholic formulations.
The water activity of a sweetener influences product microbial
stability and freshness. Fructose has a lower water activity and a
higher osmotic pressure than sucrose. Syrup formulations may be
made at lower dry-substance levels than sugar syrups without
compromising shelf-life stability. It may be necessary to include a
thickener or gelling agent to match the texture or viscosity of the
sugar-equivalent formulation.
Fructose is sweeter than the sugar alcohols mannitol and
sorbitol, which are commonly used as tableting excipients.
Although fructose is effective at masking unpleasant flavors in
tablet formulations, tablets of satisfactory hardness and friability
can only be produced by direct compression if tablet presses are
operated at relatively slow speeds. However, by the combination of
crystalline fructose with tablet-grade sorbitol in a 3 : 1 ratio,
satisfactory direct-compression characteristics can be achieved. A
directly compressible grade of fructose, containing a small amount
of starch (Advantose FS 95, SPI Pharma) is also commercially
available. Pregranulation of fructose with 3.5% povidone also
produces a satisfactory tablet excipient.(1) The added sweetness of
fructose may also be used to advantage by coating the surface of
chewable tablets, lozenges, or medicinal gums with powdered
fructose.
The coprecipitation of fructose with hydrophobic drugs such as
digoxin has been shown to enhance the dissolution profile of such
drugs. Fructose apparently acts as a water-soluble carrier upon
coprecipitation, thereby allowing hydrophobic drugs to be more
readily wetted.
Biochem/physiol Actions
D-(?)-Fructose can enhance mood and gastrointestinal disturbances in fructose malabsorbers. It also possess metabolic and endocrine impact that shows that increased consumption of fructose is a contributing factor in the development of obesity and the accompanying metabolic abnormalities observed in the insulin resistance syndrome.
Safety
Although it is absorbed more slowly than dextrose from the
gastrointestinal tract, fructose is metabolized more rapidly. Metabolism
of fructose occurs mainly in the liver, where it is converted
partially to dextrose and the metabolites lactic acid and pyruvic
acid. Entry into the liver and subsequent phosphorylation is insulinindependent.
Further metabolism occurs by way of a variety of
metabolic pathways. In healthy and well regulated diabetics,
glycogenesis (glucose stored as glycogen) predominates.
Excessive oral fructose consumption (>75 g daily) in the absence
of dietary dextrose in any form (e.g. sucrose, starch, dextrin, etc.)
may cause malabsorption in susceptible individuals, which may
result in flatulence, abdominal pain, and diarrhea. Except in
patients with hereditary fructose intolerance, there is no
evidence to indicate that oral fructose intake at current levels is a
risk factor in any particular disease, other than dental caries.
Metabolism
Not Available
Storage
Fructose is hygroscopic and absorbs significant amounts of
moisture at relative humidities greater than 60%. Goods stored in
the original sealed packaging at temperatures below 25°C and a
relative humidity of less than 60% can be expected to retain stability
for at least 12 months.
Aqueous solutions are most stable at pH 3–4 and temperatures
of 4–70°C; they may be sterilized by autoclaving.
Purification Methods
Dissolve D(-)-fructose in an equal weight of water (charcoal, previously washed with water to remove any soluble material), filter and evaporate under reduced pressure at 45-50o to give a syrup containing 90% of fructose. After cooling to 40o, the syrup is seeded and kept at this temperature for 20-30hours with occasional stirring. The crystals are removed by centrifugation, washed with a small quantity of water and dried to constant weight under a vacuum over conc H2SO4. For higher purity, this material is recrystallised from 50% aqueous ethanol [Tsuzuki et al. J Am Chem Soc 72 1071 1950]. [Beilstein 31 H 321, 1 IV 4401.]
Incompatibilities
Incompatible with strong acids or alkalis, forming a brown coloration. In the aldehyde form, fructose can react with amines, amino acids, peptides, and proteins. Fructose may cause browning of tablets containing amines.
Regulatory Status
Included in the FDA Inactive Ingredients Database (oral solutions, syrup, and suspensions; rectal preparations; intravenous infusions). Included in the Canadian List of Acceptable Non-medicinal Ingredients.
Properties of D(-)-Fructose
| Melting point: | 119-122 °C (dec.)(lit.) |
| Boiling point: | 232.96°C (rough estimate) |
| alpha | -92.25 º (c=10,H2O,on dry sub.) |
| Density | 1.59 |
| refractive index | -92 ° (C=4, H2O) |
| storage temp. | room temp |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Crystals or Crystalline Powder |
| appearance | white crystals |
| pka | pKa (18°): 12.06 |
| color | White |
| PH | 5.0-7.0 (25℃, 0.1M in H2O) |
| Odor | at 100.00 %. odorless |
| optical activity | [α]20/D 93.5 to 91.0°, c = 10% in H2O |
| Water Solubility | 3750 g/L (20 ºC) |
| λmax | λ: 260 nm Amax: 0.04 λ: 280 nm Amax: 0.04 |
| Merck | 14,4273 |
| BRN | 1239004 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 57-48-7(CAS DataBase Reference) |
| NIST Chemistry Reference | «beta»-D-Fructose(57-48-7) |
| EPA Substance Registry System | D-Fructose (57-48-7) |
Safety information for D(-)-Fructose
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for D(-)-Fructose
| InChIKey | LKDRXBCSQODPBY-GWVKGMJFSA-N |
D(-)-Fructose manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran






You may like
-
 D(-)-Fructose CAS 57-48-7View Details
D(-)-Fructose CAS 57-48-7View Details
57-48-7 -
 D-Fructose extrapure CAS 57-48-7View Details
D-Fructose extrapure CAS 57-48-7View Details
57-48-7 -
 D-(-)-Fructose CAS 57-48-7View Details
D-(-)-Fructose CAS 57-48-7View Details
57-48-7 -
 D-Fructose for tissue culture CAS 57-48-7View Details
D-Fructose for tissue culture CAS 57-48-7View Details
57-48-7 -
 D-Fructose CAS 57-48-7View Details
D-Fructose CAS 57-48-7View Details
57-48-7 -
 D-Fructose, GR 99% CAS 57-48-7View Details
D-Fructose, GR 99% CAS 57-48-7View Details
57-48-7 -
 D-(-)-Fructose 98% CAS 57-48-7View Details
D-(-)-Fructose 98% CAS 57-48-7View Details
57-48-7 -
 Crystalline Fructose CAS 57-48-7View Details
Crystalline Fructose CAS 57-48-7View Details
57-48-7
