HEROIN
- CAS NO.:561-27-3
- Empirical Formula: C21H23NO5
- Molecular Weight: 369.42
- MDL number: MFCD00468072
- EINECS: 209-217-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
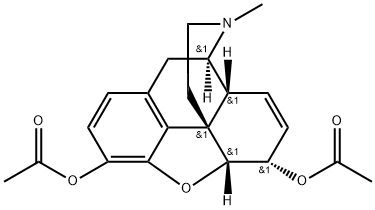
What is HEROIN?
Absorption
Bioavailability is less than 35% when orally administered . In particular, some studies have determined that the bioavailability of orally administered diamorphine could be as low as 22.9% (16.4-29.4%) on average in opioid-naive subjects .
Nevertheless, diamorphine administered by any many medically indicated routes of administration leads to a rapid absorption . Peak serum levels are achieved five to ten minutes subcutaneously, three to five minutes intranasally and intramuscularly, and less than one minute intravenously .
Toxicity
Overdosage with diamorphine well characterised by a number of symptoms including respiratory depression, pulmonary oedema, muscle flaccidity, coma or stupor, constricted pupils, cold, clammy skin and occasionally bradycardia and hypotension . The antidote for heroin overdose or poisoning is naloxone .
Chemical properties
Light Brown Solid
The Uses of HEROIN
Heroin does not occur in opium. It is madefrom poppy extract. Acetylation of morphinewith acetic anhydride or acetyl chlorideproduces heroin. Heroin is not generally usedclinically. Its illicit use in the street has beenrampant.
The Uses of HEROIN
Narcotic analgesic prepared from morphine and acetic anhydride. Controlled substance (opium derivative).
Background
Diamorphine (heroin) is a narcotic analgesic that may be habit-forming. It is a controlled substance (opium derivative) listed in the U.S. Code of Federal Regulations, Title 21 Parts 329.1, 1308.11 (1987). Sale is forbidden in the United States by Federal statute. (Merck Index, 11th ed) Internationally, diamorphine is controlled under Schedules I and IV of the Single Convention on Narcotic Drugs. As heroin, it is illegal to manufacture, possess, or sell in the United States and the UK. However, under the name diamorphine, heroin is a legal prescription drug in the United Kingdom.
Indications
Diamorphine, as a prescription medication in the United Kingdom, is indicated for use in the treatment of severe pain associated with surgical procedures, myocardial infarction or pain in the terminally ill and for the relief of dyspnoea in acute pulmonary edema .
Definition
ChEBI: A morphinane alkaloid that is morphine bearing two acetyl substituents on the O-3 and O-6 positions. As with other opioids, heroin is used as both an analgesic and a recreational drug. Frequent and regular administration is associated with tolerance and ph sical dependence, which may develop into addiction. Its use includes treatment for acute pain, such as in severe physical trauma, myocardial infarction, post-surgical pain, and chronic pain, including end-stage cancer and other terminal illnesses.
Biological Functions
Heroin is the diacetyl derivative of morphine. It is not available in the United States for therapeutic use, although its use as a recreational drug is again on the rise. It is either injected or snorted (taken intranasally). It is most often cut, or diluted, with substances such as quinine, which contribute to the flash, or high. Injection of the drug leads to the eventual collapse of the vessels into which it is injected, leading to the appearance of track marks under the skin. Heroin passes rapidly into the brain and thus has a rapid onset of action. It is then metabolized to morphine. The rapid onset contributes to the abuse liability of the drug.Heroin use in pregnant women can lead to low-birth-weight babies, babies born addicted to heroin, immunosuppression, and an increased incidence of infections in both the mother and newborn; an increased incidence of AIDS also occurs.
General Description
Heroin, was first commercially synthesized in 1898 byBayer company in Germany as an alternate analgesic tomorphine. Heroin is the 3,6 diacetylated form of morphine. The laboratory researchers, which also used theacetylation process to convert salicylic acid into acetylsalicylic acid (aspirin), believed that heroin would be an effectiveanalgesic with no addictive properties. This was unfortunatelynot the case. They named the product “heroin”because it made the test subjects, including some of thechemists, feel “heroic.” With both OH groups protected asan ester, heroin can pass through the blood-brain barrierquicker than morphine and lead to the euphoric “rush” thatbecomes so addictive to addicts, especially after IV injection.Once heroin is in the brain, it is quickly metabolizedto 3-acetylmorphine, which has low to zero activity at theμ-receptor and 6-acetylmorphine, which is 2 to 3 timesmore potent at the μ-receptor than morphine.
Heroin is not available as a prescription product in theUnited States, although it is available in some countries totreat pain associated with cancer and myocardial infarctions.It remains one of the most widely used narcotics for illicitpurposes and places major economic burdens on society.
Health Hazard
Heroin is a strongly habit-forming drug. Itis more potent than its parent compound,morphine, and much more potent thanopium. The toxic effects are similar tothose of morphine. An overdose can causerespiratory failure. It is a depressant anda stimulant of the central nervous system,showing a wide range of effects, rangingfrom drowsiness to distorted perceptionsand hallucinations. Toxicological problemsfrom an overdose of heroin may also leadto coma and pinpoint pupil. The toxicityof heroin may be enhanced when it iscoadministered with cocaine. Administrationof a narcotic antagonist such as naloxone ornaltrexone may be performed for therapy.A subcutaneous lethal dose in rabbits maybe 180 mg/kg. Heroin may produce harmfulreproductive effects in animals and humans.It is a controlled substance (opiate) listedunder the U.S. Code of Federal Regulations(Title 21, Part 329.1, 1308.11, 1987).
Pharmacokinetics
The onset of heroin's effects is dependent on the method of administration. Taken orally, heroin is totally metabolized in vivo via extensive first-pass metabolism into morphine before crossing the blood-brain barrier; so the effects are the same as orally administered morphine . Take by injection, diamorphine's acetyl groups facilitate rapid crossing into the brain . Once in the brain, heroin is rapidly metabolized into morphine by removal of the acetyl groups, therefore making it a prodrug for the delivery of morphine . Subsequently, whether eliciting actions peripherally (on smooth muscle, skeletal muscle, kidney, lung, liver, or spleen tissue , for example) or on the central nervous system, it is ultimately the morphine metabolite of heroin that then binds with opioid receptors and produces the narcotic opioid effects commonly associated with the substance .
Pharmacology
Diamorphine is available for parenteral and oral use. It is more lipid soluble
than morphine, affecting distribution and tissue penetration. One advantage
over morphine is in seings where high concentrations are required in
relatively low volumes, such as palliative care. Furthermore, when lipid
solubility is important in regulating site of action (e.g. epidural, intrathecal
use), some practitioners believe that diamorphine has specific advantages
over morphine.
Diamorphine is a prodrug. It is inactive at opioid receptors but is
converted rapidly to the active metabolites 6-monoacetylmorphine (6 MAM),
morphine and M-6-G. Presence of 6-MAM in urine or salvia can be used to
differentiate between morphine and heroin (diamorphine) consumption.
Further metabolism is similar to that of morphine; similar
problems may arise in renal impairment.
Metabolism
Once administered into the body, diamorphine undergoes deacetylation via various esterase enzymes to generate active metabolites like 6-monoacetylmorphine and morphine . In particular, when administered orally, diamorphine undergoes extensive first pass metabolism .
Properties of HEROIN
| Melting point: | 154-161°C |
| alpha | D25 -166° (c = 1.49 in methanol) |
| Boiling point: | bp12 272-274° |
| Density | 1.5600 |
| refractive index | 1.5614 (estimate) |
| Flash point: | 2℃ |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | H2O: <0.2 mg/mL |
| pka | pKa 7.83 (Uncertain) |
| form | A neat solid |
| EPA Substance Registry System | Morphinan-3,6-diol, 7,8-didehydro-4,5-epoxy-17-methyl-(5.alpha.,6.alpha.)-, 3,6-diacetate (561-27-3) |
Safety information for HEROIN
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation H331:Acute toxicity,inhalation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P337+P313:IF eye irritation persists: Get medical advice/attention. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for HEROIN
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1