Heptaldehyde
Synonym(s):Heptaldehyde;Enanthaldehyde;Enanthaldehyde, Heptaldehyde;1-Heptanal;Aldehyde C7
- CAS NO.:111-71-7
- Empirical Formula: C7H14O
- Molecular Weight: 114.19
- MDL number: MFCD00007028
- EINECS: 203-898-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:33
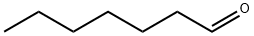
What is Heptaldehyde?
Description
Heptanal has a very strong, fatty, harsh, pungent odor and an unpleasant, fatty taste. Heptanal is obtained by distilling castor oil, preferably under reduced pressure.
Chemical properties
colourless liquid
Chemical properties
Heptanal has a very strong, fatty, harsh, pungent odor and an unpleasant, fatty taste.
Occurrence
Heptanal is a constituent of the essential oils of ylang-ylang, clary sage, California lemon, bitter orange, rose and hyacinth Also reported found in cocoa, buckwheat, elderberry fruit and juice and babaco fruit (Carica pentagona Heilborn)
The Uses of Heptaldehyde
Labelled Heptanal. Bioconversion of heptanal to heptanol by Saccharomyces cerevisiae and effect of C source maltose.
The Uses of Heptaldehyde
Manufacture of 1-heptanol; ethyl oenanthate.
The Uses of Heptaldehyde
Heptanal is used as a synthesis intermediate in the fragrances and flavors industry. It is the precursor to 1-heptanol, ethyl heptanoate and for certain lubricants.
Preparation
Obtained by distilling castor oil, preferably under reduced pressure.
Definition
ChEBI: Heptanal is an n-alkanal resulting from the oxidation of the alcoholic hydroxy group of heptan-1-ol to the corresponding aldehyde. An endogenous aldehyde coming from membrane lipid oxidation, it is found in the blood of lung cancer patients and has been regarded as a potential biomarker of lung cancer. It has a role as a biomarker. It is a saturated fatty aldehyde, a n-alkanal and a medium-chain fatty aldehyde.
Aroma threshold values
Detection: 3 to 60 ppb.
Synthesis Reference(s)
Journal of the American Chemical Society, 105, p. 6285, 1983 DOI: 10.1021/ja00358a017
The Journal of Organic Chemistry, 31, p. 3446, 1966 DOI: 10.1021/jo01348a534
General Description
A colorless, oily liquid with a penetrating fruity odor. Insoluble in water and less dense than water. Hence floats on water. Flash point near 141°F. Used to make perfumes and pharmaceuticals.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
Heptaldehyde may undergo exothermic self-condensation or polymerization reactions in the presence of acids. May generate flammable and/or toxic gases with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Is readily oxidized to give heptanoic acid. Can react with air to give first peroxo acids, and ultimately heptanoic acid. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The presence of stabilizers (antioxidants) retards autoxidation. Incompatible with strong oxidizers, bases and reducing agents.
Hazard
Combustible.
Health Hazard
May cause toxic effects if inhaled or absorbed through skin. Inhalation or contact with material may irritate or burn skin and eyes. Fire will produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Metabolism
Aldehyde C-7 (heptaldehyde) is readily oxidized in the animal body to the corresponding fatty acid, which then undergoes ?-oxidation and is eventually oxidized to carbon dioxide and water . Boyland was unable to detect pimelic acid in the urine of rats fed heptanal, indicating that the compound was probably completely oxidized in the body. The finding of tumour-inhibiting action by malonic acid supported the possibility of ω-oxidation leading to the formation of glutaric and malonic acids, although the intermediate pimelic acid was not isolated. Yoshida et al. found that heptanal was not utilized as an energy source by chicks when fed at 5% in the diet for 6 days, although the diet was palatable and caused no deaths. Direct evidence was obtained by Erwin & Deitrich for the oxidation in rat, monkey and bovine brain of heptanal and other aldehydes that may arise from biologically active amines in the brain. Aldehyde-oxidizing activity was present in all the areas of bovine brain studied. It was suggested that brain aldehyde dehydrogenase may be important in oxidizing aldehydes from exogenous sources.
Purification Methods
Dry n-heptaldehyde with CaSO4 or Na2SO4 and fractionally distil it under reduced pressure. More extensive purification is by precipitation as the bisulfite compound (formed by adding the aldehyde to saturated aqueous NaHSO3) which is filtered off and recrystallised from hot H2O. The crystals, after being filtered and washed well with H2O, are hydrolysed by adding 700mL of aqueous Na2CO3 (12.5% w/w of anhydrous Na2CO3) per 100g of aldehyde. The aldehyde is then steam distilled off, separated, dried with CuSO4 and distilled under reduced pressure in a slow stream of nitrogen. [McNesby & Davis J Am Chem Soc 76 2148 1954, Beilstein 1 H 695, 1 I 357, 1 II 750, 1 III 2844, 1 IV 3314.]
Properties of Heptaldehyde
| Melting point: | -43 °C (lit.) |
| Boiling point: | 153 °C (lit.) |
| Density | 0.817 g/mL at 25 °C (lit.) |
| vapor pressure | 3 hPa (20 °C) |
| FEMA | 2540 | HEPTANAL |
| refractive index | n |
| Flash point: | 95 °F |
| storage temp. | Flammables area |
| solubility | 1.25g/l insoluble |
| form | Powder, Crystals or Chunks |
| color | White to light yellow-beige |
| Odor | Green-herbaceous, vegetable-like odor |
| Odor Threshold | 0.00018ppm |
| explosive limit | 1.1-5.2%(V) |
| Water Solubility | insoluble |
| Sensitive | Hygroscopic |
| Merck | 14,4658 |
| JECFA Number | 95 |
| BRN | 1560236 |
| Dielectric constant | 9.1(Ambient) |
| Stability: | Stable. May be light sensitive. Flammable - readily forms explosive mixtures with air. Incompatible with strong oxidizing agents, strong bases, strong reducing agents. |
| CAS DataBase Reference | 111-71-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Heptanal(111-71-7) |
| EPA Substance Registry System | Heptanal (111-71-7) |
Safety information for Heptaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H315:Skin corrosion/irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Heptaldehyde
Heptaldehyde manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 111-71-7 99%View Details
111-71-7 99%View Details
111-71-7 -
 Heptanal CAS 111-71-7View Details
Heptanal CAS 111-71-7View Details
111-71-7 -
 Heptanal CAS 111-71-7View Details
Heptanal CAS 111-71-7View Details
111-71-7 -
 Heptaldehyde CAS 111-71-7View Details
Heptaldehyde CAS 111-71-7View Details
111-71-7 -
 Heptaldehyde 97% For Synthesis CAS 111-71-7View Details
Heptaldehyde 97% For Synthesis CAS 111-71-7View Details
111-71-7 -
 Heptaldehyde CAS 111-71-7View Details
Heptaldehyde CAS 111-71-7View Details
111-71-7 -
 Aldehyde C-7, 5 KGS, 111-71-7View Details
Aldehyde C-7, 5 KGS, 111-71-7View Details
111-71-7 -
 96% Technical Grade HeptaldehydeView Details
96% Technical Grade HeptaldehydeView Details
111-71-7
