Heptafluorobutyric acid
Synonym(s):Heptafluorobutyric acid;HFBA;Perfluorobutyric acid;HFBA solution;Perfluorobutbutyric acid solution
- CAS NO.:375-22-4
- Empirical Formula: C4HF7O2
- Molecular Weight: 214.04
- MDL number: MFCD00004171
- EINECS: 206-786-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
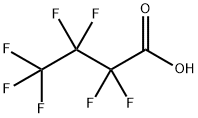
What is Heptafluorobutyric acid?
Chemical properties
Clear colorless to faintly yellow liquid
The Uses of Heptafluorobutyric acid
Heptafluorobutyric acid (HBFA) has been used widely for purifying neuropeptides because of its excellent resolution. The use of heptafluorobutyric acid as a volatile ion-pair reagent in the high-performance liquid chromatographic isolation of SB-223070 John K.
Heptafluorobutyric acid is a strong acid and ion-pairing agent that is used in analytical chemistry, notably in HPLC and in gas chromatography/mass spectrometry (GC/MS), in a similar manner to trifluoroacetic acid (TFA). The strong acidity of HFBA ensures that other acidic groups such as carboxylic acid moieties on biomolecules remain protonated, and thus the biomolecule samples are able to interact with organic solvents in such processes as reverse phase chromatography. The longer alkyl chain of HFBA makes it more hydrophobic than TFA, and thus HFBA can be utilized with more hydrophobic samples.
HFBA (0.1%) has been used in the mobile phase of an HPLC/LC-MS protocol for the detection of marine bacterioplankton sideophores.A study of the effects of various acids, including HFBA, on the resolution of intact proteins by reversed-phase LC/ESI-MS has been published.A modified version of the peptide ladder sequencing technique that incorporates allyl isothiocyanate and HFBA has been reported.
The Uses of Heptafluorobutyric acid
Heptafluorobutyric acid is an ion pair reagent for reverse-phase HPLC. It is used in the sequencing, synthesis, and solubilizing of proteins and peptides. It is used as mobile phase modifier for enhancement of selectivity in the HPLC analysis of histone proteins. It is an effective additive for zinc electrodeposition.
The Uses of Heptafluorobutyric acid
Perfluorobutyric acid was used as mobile phase modifier for enhancement of selectivity in the HPLC analysis of histone proteins. It was used as ion pairing reagent in preparative and analytical reserved phase HPLC of underivatised peptides.
What are the applications of Application
Heptafluorobutyric acid is an anion-pairing agent for reverse phase HPLC purification of proteins & peptides
Definition
ChEBI: A monocarboxylic acid that is perfluorinated butyric acid.
General Description
Perfluorobutyric acid is an effective additive for zinc electrodeposition.
Hazard
Irritant to tissue.
Purification Methods
Fractionally distil the acid twice in an Oldershaw column (p 10) with an automatic vapour-dividing head, the first distillation being in the presence of conc H2SO4 as a drying agent. (Take care with the hot acid.) [Beilstein 2 IV 810.]
Properties of Heptafluorobutyric acid
| Melting point: | -17.5 °C |
| Boiling point: | 120 °C755 mm Hg(lit.) |
| Density | 1.645 g/mL at 25 °C(lit.) |
| vapor density | 7 (vs air) |
| vapor pressure | ~10 mm Hg ( 25 °C) |
| refractive index | n |
| Flash point: | 120-121°C |
| storage temp. | Store below +30°C. |
| solubility | Chloroform: soluble; Methanol: soluble |
| pka | pK1:0.17 (25°C) |
| form | Powder |
| color | Clear colorless to faint yellow |
| Specific Gravity | approximate 1.6 |
| PH | 1 (H2O, 20℃) |
| Water Solubility | miscible |
| Sensitive | Light Sensitive |
| BRN | 1426882 |
| CAS DataBase Reference | 375-22-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, heptafluoro-(375-22-4) |
| EPA Substance Registry System | Heptafluorobutyric acid (375-22-4) |
Safety information for Heptafluorobutyric acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Heptafluorobutyric acid
| InChIKey | YPJUNDFVDDCYIH-UHFFFAOYSA-N |
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 Heptafluorobutyric acid 98%View Details
Heptafluorobutyric acid 98%View Details -
 Heptafluorobutyric Acid CAS 375-22-4View Details
Heptafluorobutyric Acid CAS 375-22-4View Details
375-22-4 -
 Heptafluorobutyric acid 98% CAS 375-22-4View Details
Heptafluorobutyric acid 98% CAS 375-22-4View Details
375-22-4 -
 Heptafluorobutyric acid, 99% CAS 375-22-4View Details
Heptafluorobutyric acid, 99% CAS 375-22-4View Details
375-22-4 -
![Heptafluorobutyric Acid [Sequenation Reagent] CAS 375-22-4](https://img.chemicalbook.in//Content/image/CP5.jpg) Heptafluorobutyric Acid [Sequenation Reagent] CAS 375-22-4View Details
Heptafluorobutyric Acid [Sequenation Reagent] CAS 375-22-4View Details
375-22-4 -
![Heptafluorobutyric Acid (ca. 0.5mol/L in Water) [Ion-Pair Reagent for LC-MS] CAS 375-22-4](https://img.chemicalbook.in//Content/image/CP5.jpg) Heptafluorobutyric Acid (ca. 0.5mol/L in Water) [Ion-Pair Reagent for LC-MS] CAS 375-22-4View Details
Heptafluorobutyric Acid (ca. 0.5mol/L in Water) [Ion-Pair Reagent for LC-MS] CAS 375-22-4View Details
375-22-4 -
 Heptafluorobutyric acid CAS 375-22-4View Details
Heptafluorobutyric acid CAS 375-22-4View Details
375-22-4 -
 Heptafluorobutyric acid CAS 375-22-4View Details
Heptafluorobutyric acid CAS 375-22-4View Details
375-22-4
