guanidine
- CAS NO.:113-00-8
- Empirical Formula: CH5N3
- Molecular Weight: 59.07
- EINECS: 204-021-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
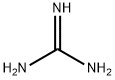
What is guanidine?
Absorption
Rapidly absorbed and distributed
Toxicity
LD50 = 475 mg/kg (oral, rat). Can cause severe gastrointestinal symptoms (nausea, vomiting and diarrhea), bone marrow suppression, renal insufficiency and other hematologic abnormalities (anemia, leucopenia). Severe guanidine intoxication is characterized by nervous hyperirritability, fibrillary tremors and convulsive contractions of muscle, salivation, vomiting, diarrhea, hypoglycemia, and circulatory disturbances.
Description
Guanidine is a small, nitrogen-rich organic compound found in nature in plants (e.g., rice hulls and turnip juice) and animals (e.g., mussels and earthworms). Unlike its oxygen analogue, carbonic acid, it exists at ambient conditions (i.e., not in solution nor at cryogenic temperatures). Guanidine should not be confused with guanine, a purine derivative that is found in bat and bird feces.
Many literature sources state that guanine is very soluble in water; but, as shown in the fast facts table, it is only slightly soluble. It is, however, very hygroscopic. Its hydrochloride salt is highly water-soluble and is the usual article of commerce. The free base is extremely toxic, as shown in the hazard information table.?
Guanidine was discovered in nature in the late 19th century. In 1907, a German patent was awarded to Italian chemist Celso Ulpiani for the reaction of dicyanamide with strong acid to produce guanidine (actually, guanidinium) nitrate. Twenty-four years later, G.B.L. Smith, V. J. Sabetta, and O. F. Steinbach, Jr., at the Polytechnic Institute of Brooklyn (now New York University Tandon School of Engineering) published a comprehensive study of the conversion of dicyandiamide (aka 2-cyanoguanidine) to guanidinium nitrate and then to nitroguanidine, a powerful explosive.
Guanidine’s hydrothiocyanate salt has an interesting connection to the current COVID-19 pandemic. This past June, the US Food and Drug Administration warned laboratories not to use media that contain the thiocyanate salt or other guanidine-based chemicals to transport COVID-19 samples. The reason: Guanidine compounds can decompose to highly toxic hydrogen cyanide (HCN) gas. The FDA also advised that when personnel do not know the ingredients of a transport medium, they should handle it as though it contained a guanidine product. As of the date of FDA’s warning, no HCN-related injuries had been reported to the agency.
Chemical properties
Colorless crystals. Soluble inwater and alcohol. Combustible. Guanidine is a strong monobasic Bronsted base, which absorbs water and carbon dioxide from the air and forms strongly alkaline solutions with water and alcohols; the pH of a 20 wt % aqueous solution is ca. 13.5 (25 ℃). In aqueous solution at elevated temperature guanidine is hydrolyzed to urea; airtight alcohol solutions are stable. Guanidine is infinitely soluble in water, ethanol, methanol, and dimethylformamide.
The Uses of guanidine
Guanidine is an intermediate in synthesizing N-Formylguanidine (F700505), which is used in the preparation of Amiloride (A578700), a sodium channel blocker used as a diuretic drug.
The Uses of guanidine
Organic synthesis
Indications
For the reduction of the symptoms of muscle weakness and easy fatigability associated with the myasthenic syndrome of Eaton-Lambert. It is not indicated for treating myasthenia gravis.
Background
A strong organic base existing primarily as guanidium ions at physiological pH. It is found in the urine as a normal product of protein metabolism. It is also used in laboratory research as a protein denaturant. (From Martindale, the Extra Pharmacopoeia, 30th ed and Merck Index, 12th ed) It is also used in the treatment of myasthenia and as a fluorescent probe in HPLC.
Production Methods
Guanidine is formed (1) by heating ammonium thiocyanate to 180 °C, (2) by ammonolysis of orthocarbonates, C(OC2H5)4 + 3NH3 → (NH2)2C=NH + 4C2H5OH, (3) by ammonolysis of chloropicrin, Cl3CNO2 + 7NH3 → NH2)2 C=NH + 3NH4Cl + N2 + 3H2O, (4) by ammonolysis of cyanogen chloride, ClCN + NH3 → ClC(NH2)=NH → HN=C=NH → (NH2)2 C=NH.
Guanidine forms salts with acids, e.g., guanidine nitrate, HNC(NH2)2 · HNO3. By heating at 120 °C for several hours, a mixture of ammonium thiocyanate and dicyanodiamide, guanidine thiocyanate solution is obtained by extracting with water. Treating guanidine with a mixture of nitric and sulfuric acids forms nitroguanidine which is reduced by zinc and acetic acid to aminoguanidine.
By treating aminoguanidine (1) with dilute acid or alkali, there is obtained, first, semicarbazide, finally hydrazine; (2) with nitrous acid, diazoguanidine which is decomposed by alkali into alkali azide (e.g., NaN3) plus cyanamide (H2N·CN) plus water.
Definition
ChEBI: Guanidine is an aminocarboxamidine, the parent compound of the guanidines. It is a one-carbon compound, a member of guanidines and a carboxamidine. It is a conjugate base of a guanidinium.
Agricultural Uses
Carbamidine is another name for guanidine. It is a crystalline basic compound related to urea. It forms a number of salts which are used as fertilizers. Guanidine phosphate is an example.
Pharmacokinetics
Guanidine apparently acts by enhancing the release of acetylcholine following a nerve impulse. It also appears to slow the rates of depolarization and repolarization of muscle cell membranes.
Metabolism
Not metabolized.
Purification Methods
Crystallise it from water/EtOH under nitrogen. It is very deliquescent and absorbs CO2 from the air readily. [Jones Trans Faraday Soc 55 524 1959, Beilstein 3 H 82, 3 I 39, 3 II 69, 3 III 154, 3 IV 148.]
Properties of guanidine
| Melting point: | ~50° |
| Boiling point: | 82.3°C (rough estimate) |
| Density | 1.6000 (rough estimate) |
| refractive index | 1.5950 (estimate) |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| appearance | hygroscopic white crystals |
| pka | pKa ~12.5(at 25℃) |
| color | White |
| EPA Substance Registry System | Guanidine (113-00-8) |
Safety information for guanidine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P391:Collect spillage. Hazardous to the aquatic environment P337+P313:IF eye irritation persists: Get medical advice/attention. P501:Dispose of contents/container to..… |
Computed Descriptors for guanidine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1