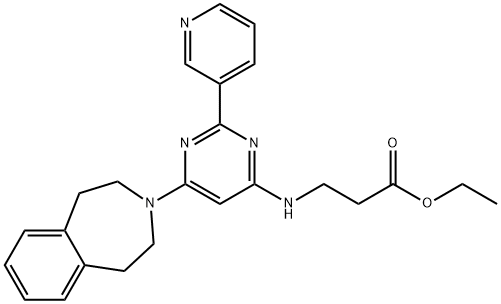
GSK J4 HCl
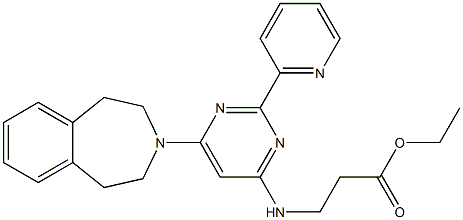
Synonym(s):Ethyl 3-((6-(4,5-dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(pyridin-2-yl)pyrimidin-4-yl)amino)propanoate;GSK-J1 Pro-Drug, JHDM Inhibitor II Pro-Drug, Ethyl-3-(6-(4,5-dihydro-1H-benzo[d]azepin-3(2H)-yl)-2-(pyridin-2-yl)pyrimidin-4-ylamino)propanoate;Histone Lysine Demethylase Inhibitor VIII, GSK-J4 - CAS 1373423-53-0 - Calbiochem
- CAS NO.:1373423-53-0
- Empirical Formula: C24H27N5O2
- Molecular Weight: 417.5
- MDL number: MFCD22683852
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-05 21:24:55
What is GSK J4 HCl?
Description
GSK-J4 (1373423-53-0) is a histone demethylase JMJD3/UTX inhibitor. Inhibits demethylation of histone H3K27. Reduces LPS-induced proinflammatory cytokine production in primary human macrophages (IC50 = 9 μM for the inhibition of TNFα release). Cell permeable, ethyl ester of GSK J1 (cat.# 10-1393).1 GSK-J4 rescues newborn pups from embryonic lethality in BRAF knockin mice which recapitulate major features of RASopathies.2
The Uses of GSK J4 HCl
GSK-J4 has been used to study the effect of KDM2B (Jumonji (JmjC) domain histone 3 lysine 36 (H3K36) di-demethylase) inhibition on the survival and DNA repair potential of glioblastoma cells. It has also been used in sulforhodamine B (SRB) cell growth assay and cell viability assay.
What are the applications of Application
GSK J4 is A cell permeable inhibitor of the histone demethylase JMJD3/UTX
Definition
ChEBI: 3-[[2-(2-pyridinyl)-6-(1,2,4,5-tetrahydro-3-benzazepin-3-yl)-4-pyrimidinyl]amino]propanoic acid ethyl ester is an organonitrogen heterocyclic compound.
Biochem/physiol Actions
GSK-J4 is cell permeable prodrug rapidly hydrolysed by macrophage esterases to GSK-J1, a potent selective jumonji H3K27 demethylase inhibitor. Jumonji C domain-containing histone demethylases (JHDMs) are Fe(II) and α-ketoglutarate dependent enzymes that oxygenate methylated histone lysine residues and thereby cause their demethylation. GSK-J1 is selective for the KDM6 subfamily members JMJD3 and UTX with an IC50 of 60 nM in a JMJD3 assay, and is inactive against other demethylases of the JMJ family and over 100 tested kinases and histone deacetylases. The prodrug GSK-J4 inhibited TNF-α production with an IC50 of 9 μM in LPS-stimulated human macrophages and blocked the production of TNF-α by macrophages derived from patients with rheumatoid arthritis. For characterization details for GSK-J4 and full characterization details for GSK-J1, please visit the GSK-J1 probe summary on the Structural Genomics Consortium (SGC) website.To learn about other SGC chemical probes for epigenetic targets, visit sigma.com/sgc
storage
Room temperature
References
1) Kruidenier et al. (2012), Selective jumonji H3K27 demethylase inhibitor modulates the proinflammatory macrophage response; Nature, 488 404 2) Inoue et al. (2014) New Braf knockin mice provide a pathogenic mechanism of developmental defects and therapeutic approach in cardio-facio-cutaneous syndrome; Hum. Mol. Genet.,?23?6553
Properties of GSK J4 HCl
| Boiling point: | 581.2±50.0 °C(Predicted) |
| Density | 1.216±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: soluble20mg/mL, clear |
| pka | 5.95±0.10(Predicted) |
| form | Tan semi-solid |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 3 months. |
Safety information for GSK J4 HCl
Computed Descriptors for GSK J4 HCl
| InChIKey | WBKCKEHGXNWYMO-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![(2R,3R,4S,5R)-2-(6-aMino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]iMidazol-2-yl)ethyl)cyclobutyl)(isopropyl)aMino)Methyl)tetrahydrofuran-3,4-diol](https://img.chemicalbook.in/CAS/20150408/GIF/1380288-87-8.gif)

You may like
-
 Histone Lysine Demethylase Inhibitor VIII, GSK-J4 CAS 1373423-53-0View Details
Histone Lysine Demethylase Inhibitor VIII, GSK-J4 CAS 1373423-53-0View Details
1373423-53-0 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2