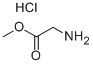
Glycine hydrochloride
Synonym(s):Aminoacetic acid;Aminoethanoic acid;Glycocoll
- CAS NO.:6000-43-7
- Empirical Formula: C2H6ClNO2
- Molecular Weight: 111.53
- MDL number: MFCD00012872
- EINECS: 227-841-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
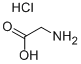
What is Glycine hydrochloride?
Chemical properties
white crystals or crystalline powder
The Uses of Glycine hydrochloride
Glycine hydrochloride is an inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.Glycine Hydrochloride is commonly used in buffer solutions, in electrophoresis, and preparative chromatography.
What are the applications of Application
Glycine hydrochloride is a spinal cord inhibitory neurotransmitter
What are the applications of Application
Glycine hydrochloride has been used as a component of Ag (silver)-stripping buffer for multiplex immunohistochemistry in mice tissues.
Glycine hydrochloride has been used:
in the preparation of glycine-HCl buffer
to elute serum immunoglobulins (IgGs) from the beads in order to check for the complete enrichment of core- and site-specific antibodies
to regenerate the sensor to elute the bound C-reactive protein (CRP)
Definition
Glycine hydrochloride is a non-essential amino acid. It is found primarily in gelatin and silk fibroin and used therapeutically as a nutrient. It is also a fast inhibitory neurotransmitter.
Preparation
Glycine hydrochloride can be prepared by the action of hydrochloric acid on hippuric acid,aminoacetonitrile,methyleneaminoacetonitrile, and on ethyl phthaliminoacetate. Aniline has been recommended for its conversion into free glycine. Glycine can also be prepared by the interaction of chloroacetic acid and ammonia; by the hydrolysis of methyleneaminoacetonitrile by successive treatments with barium hydroxide and sulfuric acid; and by the hydrolysis of aminoacetonitrile by means of barium hydroxide. A thorough study has been reported for its preparation in improved yields by the sulfuric acid hydrolysis of aminoacetonitrile, and in satisfactory yields from chloroacetic acid and ammonia. Other methods of preparation include the reduction of cyanoformic esters, a modified Curtius degradation of ethyl cyanoacetate, and the hydrolysis of the neck ligaments of cattle.
synthesis of Glycine hydrochloride
Flammability and Explosibility
Not classified
Biochem/physiol Actions
Glycine acts on receptors which bring out an enhanced chloride conductance. It shows highest concentrations in the spinal cord as compared to elsewhere in the brain. However, it interacts with and allostericaly activates the excitatory receptor N-methyl-D-aspartate (NMDA), thus, resulting in excitatory transmission in brain.
Glycine comprises a single carbon molecule, that is attached to the amino and a carboxyl group. Glycine hydrochloride (Gly·HCl) is capable of liquifying native chitosan and also helps in the preparation of regenerated chitosan membrane. It can serve as an osmoprotectant. Glycine can act as a flexible link in proteins and can also allow the formation of helices. Free glycine may have a protecting role in tissues against ischemia, hypoxia and reperfusion.
Inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.
Purification Methods
Crystallise the salt from absolute EtOH or 80% EtOH. Monoglycine hydrochloride has m 176-177o, and diglycine monohydrochloride has m 187o. [Frost J Am Chem Soc 64 1286 1942, Beilstein 4 III 1111, 4 IV 2353.]
Properties of Glycine hydrochloride
| Melting point: | 176-180 °C(lit.) |
| Density | 1.457[at 20℃] |
| vapor pressure | 0.003Pa at 20℃ |
| storage temp. | 2-8°C |
| form | Crystalline Powder |
| color | White to almost white |
| Water Solubility | SLIGHTLY SOLUBLE |
| Sensitive | Hygroscopic |
| Merck | 14,4491 |
| BRN | 3909617 |
| CAS DataBase Reference | 6000-43-7(CAS DataBase Reference) |
| EPA Substance Registry System | Glycine, hydrochloride (6000-43-7) |
Safety information for Glycine hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Glycine hydrochloride
Glycine hydrochloride manufacturer
Paras Intermediates Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Glycine hydrochloride 99% (HPLC) CAS 6000-43-7View Details
Glycine hydrochloride 99% (HPLC) CAS 6000-43-7View Details
6000-43-7 -
 Glycine Hydrochloride CAS 6000-43-7View Details
Glycine Hydrochloride CAS 6000-43-7View Details
6000-43-7 -
 Glycine hydrochloride CAS 6000-43-7View Details
Glycine hydrochloride CAS 6000-43-7View Details
6000-43-7 -
 Glycine hydrochloride CAS 6000-43-7View Details
Glycine hydrochloride CAS 6000-43-7View Details
6000-43-7 -
 Glycine hydrochloride solution CAS 6000-43-7View Details
Glycine hydrochloride solution CAS 6000-43-7View Details
6000-43-7 -
 6000-43-7 Glycine hydrochloride 98%View Details
6000-43-7 Glycine hydrochloride 98%View Details
6000-43-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1