Glutaric acid
Synonym(s):Glutaric acid;Pentanedioic acid
- CAS NO.:110-94-1
- Empirical Formula: C5H8O4
- Molecular Weight: 132.11
- MDL number: MFCD00004410
- EINECS: 203-817-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
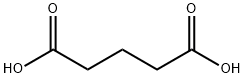
What is Glutaric acid?
Description
Glutaric acid, a five-carbon dicarboxylic acid, is a by-product of adipic acid production. It possesses carboxylic acid functional groups at both ends of its molecular chain. The terminal carboxyl group can react with primary amines in the presence of an activating agent (such as EDC or HATU) to form a stable amide bond.
Chemical properties
white or off-white crystals, usually containing 1mol crystal water. Soluble in water, alcohol, ether and chloroform, slightly soluble in petroleum ether.
Occurrence
Glutaric acid occurs in washings from fleece and, together with malonic acid, in the juice of unripened sugar beet.
The Uses of Glutaric acid
Glutaric acid is used as the raw material for organic synthesis, pharmaceutical intermediate and synthetic resin. It serves as a precursor in the production of polyester polyols, polyamides, ester plasticizers and corrosion inhibitors. It is useful to decrease polymer elasticity and in the synthesis surfactants and metal finishing compounds. It acts as an intermediate during the catabolism of lysine in mammals.
Definition
ChEBI: glutaric acid is an alpha,omega-dicarboxylic acid that is a linear five-carbon dicarboxylic acid. It has a role as a human metabolite and a Daphnia magna metabolite. It is an alpha,omega-dicarboxylic acid and a dicarboxylic fatty acid. It is a conjugate acid of a glutarate(1-) and a glutarate.
Preparation
glutaric acid is produced as a by-product of the production process of adipic acid (about 2% of the output of an adipic acid plant is glutaric); Glutaric acid can be produced through various chemical routes, for example, from cyclopentane by oxidation with molecular oxygen and cobalt (III) catalysts or by ozonolysis; and from cyclopentanol–cyclopentanone by oxidation with oxygen and Co(CH3CO2)2, with potassium peroxide in benzene, or with N2O4 or nitric acid. Together with succinic acid, glutaric acid is formed as a by-product during oxidation of cyclohexanol–cyclohexanone in the adipic acid production process.
What are the applications of Application
The applications of glutaric acid, e.g., as an intermediate, are limited. Its use as a starting material in the manufacture of maleic acid has no commercial importance. it does not usually reach the market because it is converted into dibasic esters and sold as environmentally friendly solvents.
Synthesis
Glutaric acid is synthesised from α-ketoglutaric acid, with hydrogenation of the intermediate 1,3-dithiolane diamine serving as the key step. α-Ketoglutaric acid itself is produced from glucose via fermentation using Glutamic acid bacillus GKGA.
Synthesis Reference(s)
Journal of the American Chemical Society, 78, p. 2489, 1956 DOI: 10.1021/ja01592a042
Organic Syntheses, Coll. Vol. 1, p. 290, 1941
Synthetic Communications, 10, p. 205, 1980 DOI: 10.1080/00397918008064223
Fire Hazard
Flash point data for 1,5-Pentanedioic acid are not available; however, 1,5-Pentanedioic acid is probably combustible.
Flammability and Explosibility
Not classified
Purification Methods
Crystallise the acid from *benzene, CHCl3, distilled water or *benzene containing 10% (w/w) of diethyl ether. Dry it under vacuum. [Beilstein 2 IV 1934.]
Properties of Glutaric acid
| Melting point: | 95-98 °C (lit.) |
| Boiling point: | 200 °C/20 mmHg (lit.) |
| Density | 1,429 g/cm3 |
| vapor pressure | 0.022 hPa (18.5 °C) |
| refractive index | nD106 1.41878 |
| Flash point: | 200°C/20mm |
| storage temp. | Store below +30°C. |
| solubility | water: soluble5mg/mL, clear to slightly hazy, colorless to faintly yellow |
| form | Crystalline Powder |
| pka | 4.31(at 25℃) |
| color | Orange |
| PH | 3.7(1 mM solution);3.17(10 mM solution);2.66(100 mM solution) |
| Water Solubility | 430 g/L (20 ºC) |
| Merck | 14,4473 |
| BRN | 1209725 |
| Stability: | Stable. Incompatible with bases, oxidizing agents, reducing agents. |
| CAS DataBase Reference | 110-94-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Pentanedioic acid(110-94-1) |
| EPA Substance Registry System | Pentanedioic acid (110-94-1) |
Safety information for Glutaric acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P363:Wash contaminated clothing before reuse. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Glutaric acid
| InChIKey | JFCQEDHGNNZCLN-UHFFFAOYSA-N |
Glutaric acid manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Glutaric acid 98%View Details
Glutaric acid 98%View Details
110-94-1 -
 Glutaric acid, 99% CAS 110-94-1View Details
Glutaric acid, 99% CAS 110-94-1View Details
110-94-1 -
 Glutaric Acid pure CAS 110-94-1View Details
Glutaric Acid pure CAS 110-94-1View Details
110-94-1 -
 Glutaric acid 98% CAS 110-94-1View Details
Glutaric acid 98% CAS 110-94-1View Details
110-94-1 -
 Glutaric Acid (ca. 50% in Water, ca. 4.3mol/L) CAS 110-94-1View Details
Glutaric Acid (ca. 50% in Water, ca. 4.3mol/L) CAS 110-94-1View Details
110-94-1 -
 Glutaric Acid CAS 110-94-1View Details
Glutaric Acid CAS 110-94-1View Details
110-94-1 -
 Glutaric Acid CASView Details
Glutaric Acid CASView Details -
 GLUTARIC ACID For Synthesis CAS 110-94-1View Details
GLUTARIC ACID For Synthesis CAS 110-94-1View Details
110-94-1
