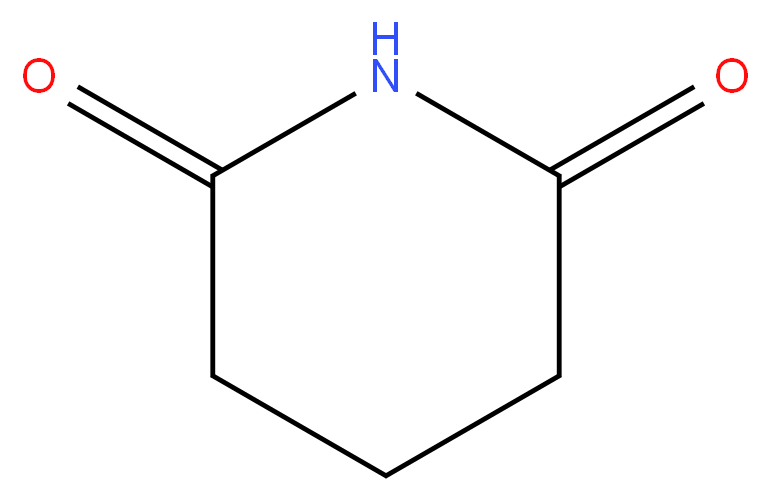
Glutarimide
Synonym(s):2,6-Piperidinedione;NSC 58190
- CAS NO.:1121-89-7
- Empirical Formula: C5H7NO2
- Molecular Weight: 113.11
- MDL number: MFCD00006670
- EINECS: 214-340-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-06 08:32:39
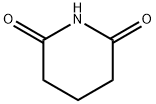
What is Glutarimide?
Description
Glutarimide, also known as Piperidine-2,6-dione or 2,6-Piperidinedione, is a versatile and commonly used organic reagent, classified in pharmacology as a protein synthesis inhibitor compound. It is also an important component of the anti-cancer drug thalidomide. The study reported the synthesis of triazoloquinoxaline derivative (Compound 15), a new potent anticancer drug containing a glutarimide molecule, based on the CRBN binding property of the glutarimide molecule in thalidomide molecule and the cytotoxic activity of triazole-quinoxaline. The IC50s were 9.81 ± 0.7, 15.49 ± 1.2 and 10.09 ± 0.9 μM for hepatocellular carcinoma (HepG2), prostate cancer (PC3) and breast cancer (MCF-7), respectively. It was superior to thalidomide in decreasing NF-κB P65 levels in HepG-2 cells[1].
Chemical properties
white crystalline powder
The Uses of Glutarimide
Glutarimide acts as an inhibitor of protein synthesis. Further, it is used as a reactant for thionations and biocatalytic asymmetric synthesis of sitagliptin production. It is also employed in the generation of beta-adrenoceptor ligands, enantioselective synthesis of securinega alkaloids and alfa-fluoro-alfa amino amides. In addition to this, it is used in intramolecular amidocyclopropanation reactions.
What are the applications of Application
Glutarimide is a reactant for thionations
Definition
ChEBI: Glutarimide (Piperidine-2,6-dione) is a dicarboximide that is piperidine which is substituted by oxo groups at positions 2 and 6. It is a member of piperidones and a dicarboximide.
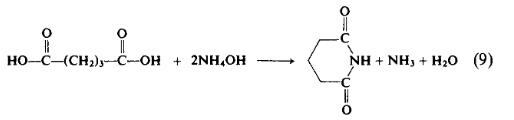
Preparation
To a flask containing 70 gm (0.53 mole) of glutaric acid is added 150 ml (2.2 mole) of 28% aqueous ammonia. The mixture is set for distillation and heated for 7 hr as the temperature of the mixture rises from 90° to 180°C. The temperature is held at 170-180°C for \\ hr or until the evolution of ammonia ceases. The reaction mixture solidifies on cooling and the product is recrystallized from acetone to afford 37.4 gm (63%), m.p. 145-146°C.
t may be advantageous to preform the ammonium salt of dicarboxylic acids prior to the application of enough heat to form the imide. The preparation of succinimide is a case in point.
General Description
A glutarimide antibiotic, 9-methylstreptimidone, shows antiviral, antitumor and antifungal activities.
Purification Methods
Purify it by dissolving 75g in 200mL of H2O, boil for 30minutes with 2g of charcoal, filter, evaporate to dryness and recystallise the residue from 125mL of 95% EtOH to give 70g of white crystals, m 152-154o. It also crystallises from Me2CO (m 163-165o) or EtOH (m 153-154o). The N-bromo derivative (a brominating agent) crystallises from H2O with m 180-185o. [Paris et al. Org Synth Coll Vol IV 496 1963, Beilstein 21 H 382, 21 I 331, 21 II 307, 21 III/IV 4582.]
References
[1] MAGED MOHAMMED SALEH AL WARD . Design, synthesis and biological evaluation of newly triazolo-quinoxaline based potential immunomodulatory anticancer molecules[J]. Journal of Molecular Structure, 2023. DOI:10.1016/j.molstruc.2023.137041.
Properties of Glutarimide
| Melting point: | 155-157 °C (lit.) |
| Boiling point: | 211.82°C (rough estimate) |
| Density | 1.2416 (rough estimate) |
| refractive index | 1.4200 (estimate) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | soluble in Chloroform, DCM |
| pka | pKa 11.4 (Uncertain) |
| form | Crystals or Crystalline Flakes |
| color | White |
| Water Solubility | Soluble in water, hot ethanol and boiling benzene. Insoluble in ether. |
| BRN | 110052 |
| InChI | InChI=1S/C5H7NO2/c7-4-2-1-3-5(8)6-4/h1-3H2,(H,6,7,8) |
| CAS DataBase Reference | 1121-89-7(CAS DataBase Reference) |
| EPA Substance Registry System | Glutarimide (1121-89-7) |
Safety information for Glutarimide
Computed Descriptors for Glutarimide
| InChIKey | KNCYXPMJDCCGSJ-UHFFFAOYSA-N |
| SMILES | N1C(=O)CCCC1=O |
Glutarimide manufacturer
Synocule Research Labs Pvt Ltd
GRK RESEARCH LABORATORIES PVT LTD
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
You may like
-
 1121-89-7 Glutarimide 98%View Details
1121-89-7 Glutarimide 98%View Details
1121-89-7 -
 piperidine-2,6-dione 1121-89-7 98%View Details
piperidine-2,6-dione 1121-89-7 98%View Details
1121-89-7 -
 Glutarimide 98% CAS 1121-89-7View Details
Glutarimide 98% CAS 1121-89-7View Details
1121-89-7 -
 Glutarimide, 98% CAS 1121-89-7View Details
Glutarimide, 98% CAS 1121-89-7View Details
1121-89-7 -
 Glutarimide 1121-89-7 98%+View Details
Glutarimide 1121-89-7 98%+View Details
1121-89-7 -
 Glutarimide CAS 1121-89-7View Details
Glutarimide CAS 1121-89-7View Details
1121-89-7 -
 Glutarimide CAS 1121-89-7View Details
Glutarimide CAS 1121-89-7View Details
1121-89-7 -
 Glutarimide CAS 1121-89-7View Details
Glutarimide CAS 1121-89-7View Details
1121-89-7
