glucosulfone
- CAS NO.:554-18-7
- Empirical Formula: C24H34N2O18S3.2Na
- Molecular Weight: 780.707
- EINECS: 209-064-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-04-23 13:52:06
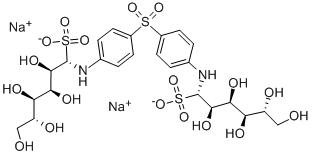
What is glucosulfone?
The Uses of glucosulfone
Medicine.
Definition
The USP grade is an aqueous solution (for injection), clear and pale yellow, p H 5.0–6.5.
Safety Profile
Poison by ingestion,subcutaneous, and intravenous routes. When heated todecomposition it emits toxic fumes of SOx, NOx, andNa2O.
Properties of glucosulfone
| color | White, amorphous powder |
| Odor | sweet-tasting solid |
Safety information for glucosulfone
Computed Descriptors for glucosulfone
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
You may like
-
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 (3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
(3-Benzyloxypropyl)triphenyl phosphonium 98%View Details
54314-85-1 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0 -
 124371-59-1 98%View Details
124371-59-1 98%View Details
124371-59-1 -
 53857-52-2 98%View Details
53857-52-2 98%View Details
53857-52-2
Statement: All products displayed on this website are only used for non medical purposes such as industrial applications or scientific research, and cannot be used for clinical diagnosis or treatment of humans or animals. They are not medicinal or edible.