Glaziovine
- CAS NO.:17127-48-9
- Empirical Formula: C18H19NO3
- Molecular Weight: 297.35
- MDL number: MFCD00865420
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
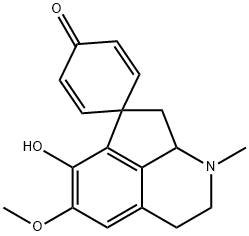
What is Glaziovine?
Originator
Suavedol,Simes,Italy,1976
Manufacturing Process
The thermal condensation of p-benzyloxyphenylacetic acid and of 3-methoxy-
4-hydroxyphenethylamine occurs and gives, with a yield of 86% to 92%, the
N-(3-methoxy-4-hydroxyphenethyl-p-benzyloxyphenyl)acetamide; from this
latter, by cyclization according to Bischler-Napieralski with phosphorus
oxychloride in acetonitrile, followed by reduction with sodium borohydride,
there is obtained with a yield of 75% to 80% the 1-(p-benzyloxybenzyl)-6-
methoxy-7-hydroxy-1,2,3,4-tetrahydroisoquinoline, which is methylated with formaldehyde and formic acid giving 1(p-benzyloxybenzyl)-2-methyl-6-
methoxy-7-hydroxy-1,2,3,4-tetrahydroisoquinoline with a yield of 90%.
This intermediate is then nitrated with 65% nitric acid. The nitro compound is
then hydrogenated to give a hydroxybenzylamino compound.
A solution of 94.2 g of 1-(p-hydroxybenzyl)-2-methyl-6-methoxy-7-hydroxy-8-
amino-1,2,3,4-tetrahydroisoquinoline in 3 liters of 1N sulfuric acid is
supplemented, with stirring, between 0°C and 5°C, with 21 grams of sodium
nitrite. The diazonium sulfate solution thus obtained is made alkaline with 2.5
liters of 2N sodium hydroxide: the diazo-oxide which is separated at the
outset as a yellow precipitate is redissolved by the excess alkali, the solution
is diluted to 10 liters with deaerated water and subjected, in a nitrogen
atmosphere at 15°C in a Pyrex glass apparatus, to the radiations of a 2,000 W
high-pressure mercury vapor lamp until the yellow hue is discharged (about
30 to 40 minutes). The solution is brought to a pH of 8.6 with hydrochloric
acid and is stirred with 1.5 liters of chloroform. The two phases are filtered,
the chloroform is separated and the aqueous phase is extracted four times
with l .5 liters of chloroform. The extracts are evaporated under reduced
pressure to a small volume and percolated through a chromatographic column
containing 1.3 kilograms of neutral alumina (activity rating IV of the
Brockmann scale). The column is then further eluted with chloroform. The
eluates are evaporated under reduced pressure and the residue is
recrystallized from ethyl acetate. There are thus obtained 40.2 grams (yield
45% of theory) of pure (+-)-glaziovine, having a melting point of 220°C to
222°C.
Therapeutic Function
Tranquilizer
Properties of Glaziovine
| Melting point: | 227-228 °C |
| Boiling point: | 470.8±45.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| pka | 9.76±0.20(Predicted) |
Safety information for Glaziovine
Computed Descriptors for Glaziovine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4