Ginsengrootextract
- CAS NO.:72480-62-7
- Molecular Weight: 0
- MDL number: MFCD04605580
- EINECS: 000-000-0
- Update Date: 2025-12-17 09:49:43
What is Ginsengrootextract?
The Uses of Ginsengrootextract
Ginsengrootextract is a redundancy because the portion of the plant used therapeutically is the root.
Definition
Extractives and their physically modified derivatives. Panax ginseng, Araliaceae.
Anticancer Research
Ginseng is a common name for a group of plants belonging to genus Panax (familyAraliaceae) which includes P. ginseng (Asian ginseng), P. notoginseng (notoginseng),and P. quinquefolius (American ginseng). The bioactive compounds of ginsengare triterpenoid glycosides or dammarane saponins which are commonlyreferred to as ginsenosides or ginseng saponins (Murthy et al. 2014a; Kim et al.2015). Ginsenosides are classified into three groups, namely, protopanaxadiol group(PPD group), protopanaxatriol group (PPT group), and ginsenoside Ro, based onthe aglycone skeleton present in them, which is derived from oleanolic acid group. Ginsenosides Rb1, Rb2, Rc, and Rd belong to PPD group, while ginsenosidesRe, Rf, Rg1, Rh1, and notoginsenoside R1 belong to the PPT group. Themajor saponins of Asian ginseng are ginsenoside Rb1, Rb2, and Rg1; those innotoginseng are ginsenosides Rb1, Rd, Rg1, and notoginsenoside R1; and Americanginseng possesses ginsenosides Rb1, Rd, and Re (Wang et al. 2015). After oral consumptionof natural ginseng, saponins are biotransformed to their metabolites byenteric microbiome before absorbed by the intestine. Usually Rb1, Re, and Rd ginsenosidesare converted into Rg3 and compound K, which exhibit potent anticanceractivity (Tawab et al. 2003; Nag et al. 2012; Li et al. 2014; Wang et al.2015). Field cultivation of ginseng generally involves 4–6 years, and accumulationof ginsenosides in cultivated plants (roots) is affected by environmental factors suchas soil, climate, and biological factors. To overcome these problems, cell and organcultures methods have been opted for rapid and large-scale production of ginsengbiomass and ginsenosides (Wu and Zhong 1999; Paek et al. 2009; Murthy et al.2014a; Thanh et al. 2014a, b; Kim et al. 2015).
Properties of Ginsengrootextract
| EPA Substance Registry System | Oils, ginseng (72480-62-7) |
Safety information for Ginsengrootextract
Computed Descriptors for Ginsengrootextract
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



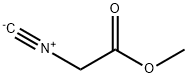
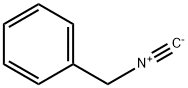
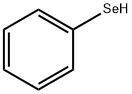
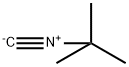
You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
