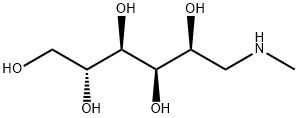
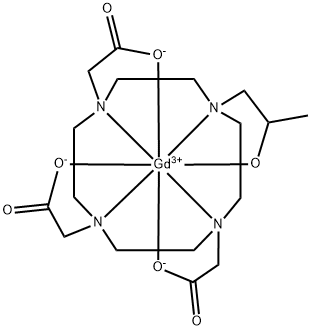
Gadobutrol
- CAS NO.:138071-82-6
- Empirical Formula: C18H31N4O9.Gd
- Molecular Weight: 604.712
- MDL number: MFCD00911649
- EINECS: 1308068-626-2
- Update Date: 2025-09-29 22:33:22
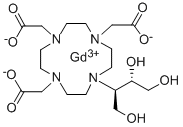
What is Gadobutrol?
The Uses of Gadobutrol
Gadobutrol is a gadolinium-based MRI contrast agent (GBCA).
The Uses of Gadobutrol
Gadobutrol injection is a magnetic resonance imaging (MRI) contrast agent that is used to help create a clear picture of the body during an MRI scan. MRI scans are a special kind of procedure that lets a doctor look at the inside of the body, such as the brain.
brand name
Gadobutrol is marketed by Bayer AG as Gadovist, and by Bayer HealthCare Pharmaceuticals as Gadavist. In India, it is also marketed by Vivere Imaging as Viv-butrol.
Mechanism of action
In the central nervous system, Gadobutrol works by highlighting any areas with disrupted blood brain barrier (BBB) and/or abnormal vascularity. In breast tissue, Gadobutrol exposes the presence and extent of malignant breast disease.
Side Effects
Side effects of Gadobutrol are uncommon but may include: headache, nausea, vomiting, feeling unwell (malaise), dizziness, abnormal or unpleasant taste in your mouth, feeling hot, numbness or tingly feeling, itching or rash, skin redness, or injection site reactions (cold feeling, warmth, pain, or burning). Its more serious side effects include: urinating less than usual or not at all; drowsiness, confusion, mood changes, increased thirst, loss of appetite; swelling, weight gain, shortness of breath; seizures (convulsions); breathing problems; pounding heartbeats or fluttering in your chest; or severe pain, burning, or irritation around the IV needle.
Dosage
Gadobutrol is given as an infusion into a vein. The recommended dose of Gadobutrol for adult and pediatric patients (including term neonates) is 0.1 mL/kg body weight (0.1 mmol/kg).
Waste Disposal
Expired or waste pharmaceuticals shall carefully take into consideration applicable DEA, EPA, and FDA regulations. It is not appropriate to dispose by flushing the pharmaceutical down the toilet or discarding to trash. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator.
Properties of Gadobutrol
| Melting point: | >264°C (dec.) |
| Density | 1.3 g/mL at 37 °C |
| storage temp. | Refrigerator |
| solubility | Water (Slightly) |
| form | Solid |
| color | White to Off-White |
| PH | 6.6-8 |
Safety information for Gadobutrol
Computed Descriptors for Gadobutrol
| InChIKey | ZPDFIIGFYAHNSK-IWEVFNEWNA-K |
| SMILES | N1(CCN(CC([O-])=O)CCN(CC([O-])=O)CCN(CC([O-])=O)CC1)[C@H](CO)[C@H](O)CO.[Gd+3] |&1:24,27,r| |
Gadobutrol manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Gadobutrol 97%View Details
Gadobutrol 97%View Details -
 Gadobutrol >98%View Details
Gadobutrol >98%View Details -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8