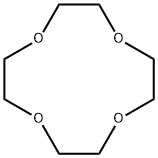
Cyclen
Synonym(s):1,4,7,10-Tetraazacyclododecane
- CAS NO.:294-90-6
- Empirical Formula: C8H20N4
- Molecular Weight: 172.27
- MDL number: MFCD00066281
- EINECS: 608-365-3;425-450-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-06 08:32:39
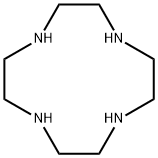
What is Cyclen?
Chemical properties
almost white to slightly yellow crystalline powder, soluble in methanol, ethanol, dmso and other organic solvents, derived from stephania cepharantha.
The Uses of Cyclen
Cyclen is an azamocrocycle, which can be used in the development of fluorescent nanosensors for the detection of metal ions.
What are the applications of Application
Cyclen is a macrocyclic aza analogue of the crown ether 12-crown-4
What are the applications of Application
Cyclen (1,4,7,10-tetraazacyclododecane) is a macrocyclic aza analogue of the crown ether 12-crown-4. Cyclen compounds are capable of selectively binding cations and are used as a ligand with chemicals used in MRI contrast (as well ass other imaging) agents.
Definition
ChEBI: 1,4,7,10-tetraazacyclododecane is an azacycloalkane that is cyclododecane in which the carbon atoms at positions 1, 4, 7 and 10 are replaced by nitrogen atoms. It is a saturated organic heteromonocyclic parent, a crown amine and an azacycloalkane.
Synthesis Reference(s)
Journal of the American Chemical Society, 96, p. 2268, 1974 DOI: 10.1021/ja00814a056
General Description
Cyclen is a microcyclic tetramine that can be used as a ligand that forms a co-ordination linkage with the surface metal cations. It can be used as a synthetic precursor. It can be prepared by S-alkylation of dithiooxamide with an excess amount of bromoethane.
Flammability and Explosibility
Not classified
Synthesis
Some syntheses exploit the Thorpe-Ingold effect to facilitate ring-formation. Illustrative is the reaction of the deprotonated tosylamides with ditosylates:
TsN(CH2CH2NTsNa)2 + TsN(CH2CH2OTs)2 → (TsNCH2CH2)4
The resulting macrocycle can be deprotected with strong acid. Base gives the tetramine.
High dilution conditions result in a low reaction rate penalty and this disadvantage is removed in an alternative procedure starting from triethylenetetraamine and dithiooxamide to a bisamidine – also a bis(imidazoline) – followed by reduction and ring expansion with DIBAL. 
In one study cyclen is covalently bonded through a propylene molecular spacer to adenine and chelated with zinc diperchlorate. This complex is able to selectively bind uracil and uridine in a 1:2 ratio both through the adenine part and cyclen part of the molecule as evidenced by mass spectrometry.
wiki/Cyclen
Properties of Cyclen
| Melting point: | 110-113 °C (lit.) |
| Boiling point: | 292.61°C (rough estimate) |
| Density | 1.0415 (rough estimate) |
| vapor pressure | 0.004Pa at 20℃ |
| refractive index | 1.5872 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 10.53±0.20(Predicted) |
| form | Crystalline Powder |
| color | Almost white to slightly yellow |
| Water Solubility | almost transparency |
| BRN | 606114 |
| Stability: | hygroscopic |
| CAS DataBase Reference | 294-90-6(CAS DataBase Reference) |
Safety information for Cyclen
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H311:Acute toxicity,dermal H314:Skin corrosion/irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Cyclen
| InChIKey | QBPPRVHXOZRESW-UHFFFAOYSA-N |
Cyclen manufacturer
HESTUR PHARMA PRIVATE LIMITED
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

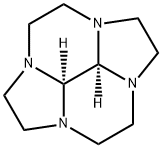
![DODECAHYDRO-3H,7BH-2A,5,5B,7A,10,10B-HEXAAZACYCLOPENTA[HI]ACEANTHRYLENE](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB9779378.gif)


You may like
-
 294-90-6 1,4,7,10-TETRAAZACYCLODODECANE 99%View Details
294-90-6 1,4,7,10-TETRAAZACYCLODODECANE 99%View Details
294-90-6 -
 1,4,7,10-tetraazacyclododecane 98%View Details
1,4,7,10-tetraazacyclododecane 98%View Details
294-90-6 -
 Tetraaza-12-crown-4 98%View Details
Tetraaza-12-crown-4 98%View Details -
 Tetraaza-12-crown-4 294-90-6 99%View Details
Tetraaza-12-crown-4 294-90-6 99%View Details
294-90-6 -
 Cyclen, 99% CAS 294-90-6View Details
Cyclen, 99% CAS 294-90-6View Details
294-90-6 -
 1,4,7,10-Tetraazacyclododecane CAS 294-90-6View Details
1,4,7,10-Tetraazacyclododecane CAS 294-90-6View Details
294-90-6 -
 Cyclen CAS 294-90-6View Details
Cyclen CAS 294-90-6View Details
294-90-6 -
 294-90-6 Cyclen 99%View Details
294-90-6 Cyclen 99%View Details
294-90-6
