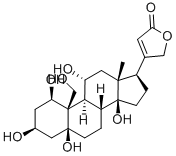
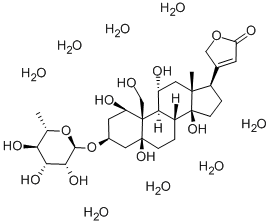
G-STROPHANTHIN
Synonym(s):1β,3β,5β,11α,14,19-Hexahydroxycard-20(22)-enolide 3-(6-deoxy-α-L -mannopyranoside);Acocantherine;G-Strophanthin;Ouabain octahydrate
- CAS NO.:630-60-4
- Empirical Formula: C29H44O12
- Molecular Weight: 584.66
- MDL number: MFCD00003688
- EINECS: 211-139-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
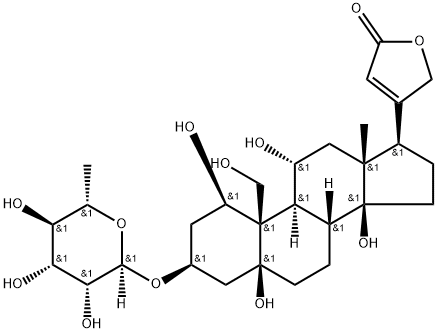
What is G-STROPHANTHIN?
Description
One of several cardiac glycosides that have been used clinically as cardiotonic agents and diuretics. Ouabain is obtained from the seeds of Strophanthus gratus, Acokanthera ouabaio, and related species. It was formerly prescribed under the name strophanthuis as the purified seed extract.
Description
Ouabain, also known as g-strophanthin, occurs in the seeds of the African plant?Strophanthus gratus, from which it was isolated by E. W. Schwartze and co-workers in 1929. It has steroid and saccharide components. Ouabain and other members of the cardenolide family present in leaves are toxic to predator insects. But many insects have developed resistance to cardenolides by mutating a single protein: ATPα.?P. Amdolfatto and colleagues at Princeton University recently discovered the mechanism.?Ouabain is used extensively in biomedical research and can be used intravenously to treat heart failure.
Chemical properties
Ouabain is a white crystalline solid.
The Uses of G-STROPHANTHIN
Ouabain is a Na+/K+-ATPase inhibitor hormone, which negatively modulates allergic airway inflammation induced by ovalbumin (OVA).
Indications
For the treatment of atrial fibrillation and flutter and heart failure
Background
A cardioactive glycoside consisting of rhamnose and ouabagenin, obtained from the seeds of Strophanthus gratus and other plants of the Apocynaceae; used like digitalis. It is commonly used in cell biological studies as an inhibitor of the NA(+)-K(+)-exchanging ATPase.
Definition
ChEBI: Ouabain is a steroid hormone that is a multi-hydroxylated alpha-L-rhamnosyl cardenoloide. It binds to and inhibits the plasma membrane Na(+)/K(+)-ATPase (sodium pump). It has been isolated naturally from Strophanthus gratus. It has a role as an EC 3.6.3.9 (Na(+)/K(+)-transporting ATPase) inhibitor, an EC 3.6.3.10 (H(+)/K(+)-exchanging ATPase) inhibitor, an EC 2.3.3.1 [citrate (Si)-synthase] inhibitor, an EC 3.1.3.41 (4-nitrophenylphosphatase) inhibitor, a plant metabolite, a cardiotonic drug, an ion transport inhibitor and an anti-arrhythmia drug. It is a cardenolide glycoside, a steroid hormone, an alpha-L-rhamnoside, a 14beta-hydroxy steroid, a 5beta-hydroxy steroid and an 11alpha-hydroxy steroid. It is a conjugate acid of an ouabain(1-).
General Description
Odorless, white crystals or crystalline powder as an octahydrate. Used to produce rapid digitalization in acute congestive heart failure. Also recommended in treatment of atrial or nodal paroxysmal tachycardia and atrial flutter.
Reactivity Profile
When heated to decomposition, G-STROPHANTHIN emits acrid smoke and fumes. Hydrolysis yields one mole ouabagenin and one mole rhamnose. Stable in air, but affected by light (G-STROPHANTHIN octahydrate) [EPA, 1998].
Health Hazard
G-STROPHANTHIN is classified as extremely toxic. Probable oral lethal dose in humans is less than 5 mg/kg or a taste (less than 7 drops) for 70 kg (150-lb.) person. Exposure may result in respiratory and cardiac failure, and/or hyperalkemia. Patients with frequent premature ventricular heart beats or who have received any preparation of digitalis during preceding three weeks are prone to toxicity.
Fire Hazard
When heated to decomposition, G-STROPHANTHIN emits acrid smoke and fumes. Hydrolysis yields one mole ouabagenin and one mole rhamnose. Stable in air, but affected by light (G-STROPHANTHIN octahydrate)
Biological Activity
Selective Na + , K + -ATPase inhibitor.
Pharmacokinetics
Ouabain, a cardiac glycoside similar to digitoxin, is used to treat congestive heart failure and supraventricular arrhythmias due to reentry mechanisms, and to control ventricular rate in the treatment of chronic atrial fibrillation.
Safety Profile
Poison by ingestion, intramuscular, intraperitoneal, intravenous, subcutaneous, and parented routes. Moderately toxic by intraduodenal route. A cardac stimulant. Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes
Potential Exposure
Ouabain, similar to digitoxin, is used to produce rapid digitalization in acute congestive heart failure. Also recommended in treatment of atrial or nodal paroxysmal tachycardia and atrial flutter; enzyme inhibitor.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Metabolism
Not Available
Storage
-20°C (desiccate)
Shipping
UN1544 Alkaloids, solid, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
It crystallises from water as the octahydrate. Dry it at 130o. It decomposes at 190o when dry. Store it in the dark as it is light sensitive, but it is stable in air. Its solubility (g/100mL) in H2O is 1.3 (~25o), 20 (~100o), and in EtOH it is 1.0 (~25o) and 12.5 (~78o). It is highly TOXIC as it is an inhibitor of cation transport and of Na+ and K+ ATPase. [Beilstein 18/5 V 625.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.).
Properties of G-STROPHANTHIN
| Melting point: | 200°C |
| Boiling point: | 561.69°C (rough estimate) |
| Density | 1.1941 (rough estimate) |
| refractive index | -32 ° (C=1, H2O) |
| storage temp. | Desiccate at -20°C |
| solubility | H2O: 10 mg/mL cold |
| form | powder |
| pka | 13.04±0.70(Predicted) |
| color | white |
| Water Solubility | 13g/L(25 ºC) |
| Merck | 6901 |
| Stability: | Hygroscopic |
| EPA Substance Registry System | Ouabain (630-60-4) |
Safety information for G-STROPHANTHIN
Computed Descriptors for G-STROPHANTHIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![OUABAIN [3H(G)]](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB4192684.gif)


You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
