FUSARIC ACID
Synonym(s):5-Butylpicolinic acid;5-Butylpyridine-2-carboxylic acid
- CAS NO.:536-69-6
- Empirical Formula: C10H13NO2
- Molecular Weight: 179.22
- MDL number: MFCD00006298
- EINECS: 208-643-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
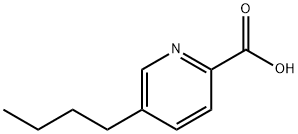
What is FUSARIC ACID?
Chemical properties
off-white to faint yellowish crystalline powder
The Uses of FUSARIC ACID
Fusaric acid may be used as a derivatizing reagent for the quantification of hydroxysteroids and dehydroepiandrosterone (DHEA)?and sulfated DHEA in biological samples using liquid chromatography electrospray-ionization-tandem mass spectrometry (LC/ESI-MS/MS) technique.
The Uses of FUSARIC ACID
A medical research tool.
What are the applications of Application
Fusaric acid is an inhibitor of dopamine β-hydroxylase
Definition
A mycotoxin and picolinic acid, that is an antibiotic and wilting agent that causes yellowing of infected plants.
Synthesis Reference(s)
The Journal of Organic Chemistry, 66, p. 605, 2001 DOI: 10.1021/jo0013554
General Description
Fusaric acid is a novel proton-affinitive derivatizing agent, having an ionization moiety and a hydrophobic moiety. It is commonly used for the derivatization of alcohols and phenols, by liquid chromatography coupled with electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS).
Hazard
Very toxic.
Biological Activity
fusaric acid is a mycotoxin produced by several species of fusarium [1]. mycotoxins are biologically active secondary fungal metabolites found as contaminants of food- and feedstuff. mycotoxin is capable of causing disease and death in both humans and animals.fusaric acid is a potent inhibitor of dopamine β-hydroxylase. fusaric acid uncompetitively inhibited the activity of dopamine β-hydroxylase with an ic50 of 30 μm in an. fusaric acid lowered endogenous levels of norepinephrine and epinephrine in brain, spleen, heart, and adrenal glands. fusaric acid inhibited dopamine β-hydroxylase activity in adrenal medulla in vivo [2]. fusaric acid altered brain and pineal neurotransmitters. in the brain and pineal gland of rats, intraperitoneally (100 mg/kg) administration of fusaric acid increased the level of 5ht, 5-hydroxyindoleacetic acid (5hiaa), tyrosine, and dopamine [3].exposure to acute doses of fusaric acid caused vomiting and neurochemical changes in swine. fusaric acid might act synergistically with trichothecene mycotoxins to cause vomiting and feed refusal in pigs consuming trichothecene-contaminated feedstuffs [4].
Safety Profile
A poison by ingestion, intraperitoneal, subcutaneous, and intravenous routes. When heated to decomposition it emits toxic fumes of NOx.
Purification Methods
Dissolve it in CHCl3, dry (Na2SO4), filter, evaporate and recrystallise the residue from 50 parts of pet ether (b 40-60o), CHCl3/pet ether or EtOAc, then sublime it in vacuo. The amide crystallises from MeOH with m 128.2-129.0o. The copper salt forms bluish violet crystals from H2O and has m 258-259o. [Hardegger & Nikles Helv Chim Acta 39 505 1956, Schreiber & Adam Chem Ber 93 1848 1960, NMR and MS: Tschesche & Führer Chem Ber 111 3500 1978, Beilstein 22 III/IV 764, 22/2 V 384.]
References
[1] hidaka h, nagatsu t, takeya k, et al. fusaric acid, a hypotensive agent produced by fungi[j]. the journal of antibiotics, 1969, 22(5): 228-230.
[2] toshiharu n, hiroyoshi h, hiroshi k, et al. inhibition of dopamine β-hydroxylase by fusaric acid (5-butylpicolinic acid) in vitro and in vivo[j]. biochemical pharmacology, 1970, 19(1): 35-44.
[3] porter j k, bacon c w, wray e m, et al. fusaric acid in fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats[j]. natural toxins, 1995, 3(2): 91-100.
[4] smith t k, macdonald e j. effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine[j]. journal of animal science, 1991, 69(5): 2044-2049.
Properties of FUSARIC ACID
| Melting point: | 96-100 °C |
| Boiling point: | 311.75°C (rough estimate) |
| Density | 1.1248 (rough estimate) |
| refractive index | 1.5710 (estimate) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | ethanol: 50 mg/mL, clear, faintly yellow |
| form | Crystalline Powder |
| pka | 1.11±0.50(Predicted) |
| color | Off-white to faint yellow |
| Merck | 14,4314 |
| BRN | 125804 |
| CAS DataBase Reference | 536-69-6(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Pyridinecarboxylic acid, 5-butyl- (536-69-6) |
Safety information for FUSARIC ACID
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for FUSARIC ACID
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 5-Butylpyridine-2-carboxylic Acid CAS 536-69-6View Details
5-Butylpyridine-2-carboxylic Acid CAS 536-69-6View Details
536-69-6 -
 Fusaric acid 95% CAS 536-69-6View Details
Fusaric acid 95% CAS 536-69-6View Details
536-69-6 -
 Fusaric acid CAS 536-69-6View Details
Fusaric acid CAS 536-69-6View Details
536-69-6 -
 Fusaric acid CAS 536-69-6View Details
Fusaric acid CAS 536-69-6View Details
536-69-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1