Furfuryl mercaptan
Synonym(s):2-Furanmethanethiol;2-Furfurylthiol;2-Furylmethanethiol;Furfuryl mercaptan
- CAS NO.:98-02-2
- Empirical Formula: C5H6OS
- Molecular Weight: 114.17
- MDL number: MFCD00003254
- EINECS: 202-628-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
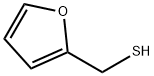
What is Furfuryl mercaptan?
Description
Furfuryl mercaptan has a characteristic unpleasant odor. It is prepared by reacting thiourea and furfuryl chloride with subsequent hydrolysis of the reaction product; also by reduction of difurfuryl disulfide in alcoholic solution using zinc dust and a small amount of acetic acid or using activated alumina. Furfuryl mercaptan tends to polymerize when heated in the presence of mineral acids.
Description
2-Furfurylthiol, which also goes by 2-furanmethanethiol, furfuryl mercaptan, and many other names, is an oily organic liquid that is best known as the principal odor component of roasting and brewing coffee.
In the late 1920s, a series of patents, primarily by noted organic chemist Hermann Staudinger1 at the University of Freiburg (Germany), described syntheses of 2-furfurylthiol. In a 1928 Canadian patent, Staudinger and co-inventor Thadeus Reichstein treated furfural with ammonium hydrogen sulfide (NH4HS) to make bis(furylmethyl) disulfide, which they then then reduced to the thiol. In a Swiss patent the same year, these inventors cited sodium sulfide and elemental zinc, aluminum, and sodium as useful reducing agents.
In a 1955 article in Organic Syntheses, Helmer Kofod at the Danish Pharmaceutical College (Copenhagen) described a synthesis of 2-furfurylthiol via the acid-catalyzed reaction of furfuryl alcohol and thiourea to produce an isothiouronium intermediate, which liberates the free thiol upon base hydrolysis.
In 1944, José Giral and Aureliano García Fernández at the University of Nuevo León (Monterrey, Mexico) were likely the first to identify 2-furfurylthiol with coffee aroma. Six years later, Staudinger and Reichstein, along with Staudinger’s son Hansjürgen, published a review of patents that covered the use of the thiol as a coffee-flavoring agent.
The Food and Agriculture Organization of the United Nations describes 2-furfurylthiol thus: “colourless oily liquid; extremely powerful and diffusive odour which on dilution becomes agreeable, coffee-like, caramellic-burnt, sweet”. In other words, the seemingly nasty compound described in the hazard information table becomes appealing when sniffed in small quantities.
Happy new year from the Molecule of the Week team—and we hope that smelling the aroma of 2-furfurylthiol on January 1 helped you wake up to the brand-new year.
1. Staudinger was awarded the 1953 Nobel Prize in Chemistry for his pioneering work on polymers.
Chemical properties
clear colorless to pale yellow liquid
Chemical properties
2-Furylmethanethiol is an important constituent of the aroma of roasted coffee. It is a liquid with an unpleasant odor, which becomes like coffee when diluted.
Chemical properties
Furfuryl mercaptan has a characteristic unpleasant odor.
Occurrence
Reported found in raw and roasted chicken, cooked beef, grilled pork, sesame seed oil, coffee and popcorn; tends to polymerize when heated in the presence of mineral acids
The Uses of Furfuryl mercaptan
Furfuryl Mercaptam is a volatile flavor component of corn tortilla chips.
Preparation
Furfuryl mercaptan is prepared from furfuryl alcohol, thiourea, and hydrogen chloride.The resulting S-furfurylisothiouronium chloride is cleaved with sodium hydroxide to give furfuryl mercaptan.
Definition
ChEBI: 2-Furanmethanethiol is a heteroarene.
Aroma threshold values
Detection: 0.005 to 0.01 ppb; aroma characteristics at 0.01%: intense sulfurous onion impact, lacrimator, slightly skunk-like with a dairy nuance and a hint of savory and coffee-like notes.
Taste threshold values
Taste characteristics at 0.2 to 1 ppb: sulfureous, roasted, onion, garlic and coffee.
General Description
Darkens on storage
Safety Profile
Poison by intraperitoneal route. Experimental reproductive effects. Used as a flavoring in chocolate, fruit, nuts, and coffee. When heated to decomposition it emits toxic fumes of SOx. See also MERCAPTANS.
Synthesis
Prepared by reacting thiourea and furfuryl chloride with subsequent hydrolysis of the reaction product; also by reduction of difurfuryl disulfde in alcoholic solution using zinc dust and a small amount of acetic acid or using activated alumina.
Properties of Furfuryl mercaptan
| Melting point: | 157.5 °C |
| Boiling point: | 155 °C(lit.) |
| Density | 1.132 g/mL at 25 °C(lit.) |
| refractive index | n |
| FEMA | 2493 | FURFURYL MERCAPTAN |
| Flash point: | 113 °F |
| storage temp. | Flammables area |
| solubility | 0.5 g/l |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly) |
| pka | 9.59±0.10(Predicted) |
| form | Liquid |
| appearance | colorless oily liquid |
| color | Clear colorless to pale yellow |
| Odor | at 0.10 % in dipropylene glycol. sulfury roasted coffee oily fatty burnt smoky |
| Water Solubility | insoluble |
| Sensitive | Air Sensitive |
| JECFA Number | 1072 |
| BRN | 383594 |
| Stability: | Air Sensitive |
| CAS DataBase Reference | 98-02-2(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Furanmethanethiol(98-02-2) |
| EPA Substance Registry System | 2-Furanmethanethiol (98-02-2) |
Safety information for Furfuryl mercaptan
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. |
Computed Descriptors for Furfuryl mercaptan
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 Furfuryl Mercaptan CAS 98-02-2View Details
Furfuryl Mercaptan CAS 98-02-2View Details
98-02-2 -
 Furfuryl mercaptan 98% CAS 98-02-2View Details
Furfuryl mercaptan 98% CAS 98-02-2View Details
98-02-2 -
 2-Furanmethanethiol CAS 98-02-2View Details
2-Furanmethanethiol CAS 98-02-2View Details
98-02-2 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 -
 7439-89-6 98.0% MinView Details
7439-89-6 98.0% MinView Details
7439-89-6 -
 Reduced Iron Powder 99.8% MaxView Details
Reduced Iron Powder 99.8% MaxView Details
7439-89-6 -
 Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
7439-89-6