Fluvoxamine maleate
Synonym(s):(E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanone-O-(2-aminoethyl)oxime maleate;Fluvoxamine maleate
- CAS NO.:61718-82-9
- Empirical Formula: C19H25F3N2O6
- Molecular Weight: 434.41
- MDL number: MFCD00269809
- EINECS: 612-212-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
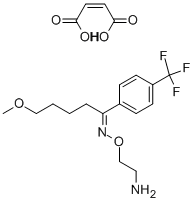
What is Fluvoxamine maleate?
Description
Fluvoxamine maleate is the most recent of the serotonin-specific antidepressants to reach the market. In vitro and in vivo animal experiments have shown fluvoxamine to have a marked effect on 5-HT mediated processes and little effect on norepinephrine. Clinical trials suggest similar efficacy to imipramine and clomipramine with a somewhat lower incidence of side effects, especially anticholinergic effects. Fluvoxamine, in contrast to the tricyclic antidepressants, does not appear to produce heart rate increase, postural hypotension or prolongation of the intraventricular conduction time and QT interval.
Chemical properties
Crystalline Solid
Originator
Duphar (Netherlands)
The Uses of Fluvoxamine maleate
Fluvoxamine maleate has been used as a test compound to determine the solubility and effective blood-brain barrier permeability.(2)
The Uses of Fluvoxamine maleate
A selective serotonin reuptake inhibitor (SSRI) used as an anti-depressant. Antiobsessional.
The Uses of Fluvoxamine maleate
estrogen
The Uses of Fluvoxamine maleate
nonsteroidal anti-inflammatory reduces pain and inflammation in eyes
The Uses of Fluvoxamine maleate
Anxiety disorder
What are the applications of Application
Fluvoxamine maleate is a SR inhibitor which binds to the 5-HT transporter
Definition
ChEBI: Fluvoxamine maleate is a member of (trifluoromethyl)benzenes.
Manufacturing Process
20.4 mmol (5.3 g) of 5-methoxy-4'-trifluoromethylvalerophenone (MP 43°C to 44°C), 20.5 mmol (3.1 g) of 2-aminooxyethylamine dihydrochloride and 10 ml of pyridine were refluxed for 15 hr in 20 ml of absolute ethanol. After evaporating the pyridine and the ethanol in vacuo, the residue was dissolved in water. This solution was washed with petroleum ether and 10 ml of 50% sodium hydroxide solution were then added. Then three extractions with 40 ml of ether were carried out. The ether extract was washed successively with 20 ml of 5% sodium bicarbonate solution and 20 ml of water. After drying on sodium sulfate, the ether layer was evaporated in vacuo. Toluene was then evaporated another three times (to remove the pyridine) and the oil thus obtained was dissolved in 15 ml of absolute ethanol. An equimolar quantity of maleic acid was added to the solution and the solution was then heated until a clear solution was obtained. The ethanol was then removed in vacuo and the residue was crystallized from 10 ml of acetonitrile at +5°C. After sucking off and washing with cold acetonitrile, it was dried in air. The MP of the resulting compound was 120°C to 121.5°C.
brand name
Luvox (Solvay Pharmaceuticals);FLOXYFRAL.
Therapeutic Function
Antidepressant
General Description
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
Hazard
A poison by ingestion. Human systemic effects.
Biological Activity
Selective serotonin reuptake inhibitor; antidepressant. Binds to the human 5-HT transporter with a K i of 1.6 nmol/l. Also available as part of the Serotonin Uptake Inhibitor Tocriset™ .
Biochem/physiol Actions
Fluvoxamine maleate is a selective neuronal serotonin reuptake inhibitor. It functions as an antidepressant and anti-obsessive agent. It is useful in treating obsessive compulsive disorder and panic disorder.
Clinical Use
SSRI antidepressant:DepressionObsessive compulsive disorder
Veterinary Drugs and Treatments
Fluvoxamine may be considered for use in treating a variety of behavior- related diagnoses in dogs and cats, including aggression and stereotypic behaviors (and other obsessive-compulsive behaviors).
Drug interactions
Potentially hazardous interactions with other drugsAminophylline and theophylline: increased aminophylline and theophylline concentrations - avoid; if not possible, halve aminophylline or theophylline dose and monitor levels.Analgesics: increased risk of bleeding with aspirin and NSAIDs; risk of CNS toxicity increased with tramadol; concentration of methadone possibly increased.Anti-arrhythmics: increased risk of toxicity with mexiletine.Anticoagulants: effect of coumarins possibly enhanced; possibly increased risk of bleeding with dabigatran.Antidepressants: avoid with reboxetine, MAOIs, moclobemide and St John’s wort; possibly enhanced serotonergic effects with mirtazapine; fluvoxamine inhibits metabolism of duloxetine - avoid; can increase tricyclics concentration; metabolism of agomelatine reduced; possible increased risk of convulsions with vortioxetine.Antiepileptics: antagonise anticonvulsant threshold; concentration of carbamazepine, fosphenytoin and phenytoin increased.Antimalarials: avoid with artemether/lumefantrine and piperaquine with artenimol.Antipsychotics: concentration of asenapine, haloperidol, clozapine and olanzapine increased; increased risk of ventricular arrhythmias with droperidol and possibly pimozide - avoid.Antivirals: concentration possibly increased by ritonavir.Ciclosporin: may increase ciclosporin concentration.Clopidogrel: possibly reduced antiplatelet effect.Cytotoxics: concentration of pomalidomide increasedDapoxetine: possible increased risk of serotonergic effects - avoid.Dopaminergics: increased risk of CNS toxicity with rasagiline - avoid; hypertension and CNS excitation with selegiline - avoid. 5HT1 agonists: risk of CNS toxicity increased with sumatriptan; possibly increased risk of serotonergic effects with naratriptan; inhibits metabolism of frovatriptan; possibly inhibits metabolism of zolmitriptan - reduce zolmitriptan dose.Linezolid: use with care, possibly increased risk of side effects.Lithium: increased risk of CNS effects - monitor levels.Melatonin: concentration of melatonin increased - avoid.Methylthioninium: risk of CNS toxicity - avoid if possible.Muscle relaxants: increased risk of toxicity with tizanidine - avoidPirfenidone: concentration of pirfenidone increased - avoid.
Metabolism
Fluvoxamine undergoes extensive hepatic transformation by CYP2D6, mainly via oxidative demethylation, into at least 9 metabolites. The 2 major metabolites showed negligible pharmacological activity. The other metabolites are not expected to be pharmacologically active.Excretion is mainly in the urine; about 2% of a dose is excreted as unchanged drug.
storage
+4°C (desiccate)
Properties of Fluvoxamine maleate
| Melting point: | 120-121.5 °C |
| Flash point: | 9℃ |
| storage temp. | 2-8°C |
| solubility | H2O: soluble |
| form | solid |
| color | White to Off-White |
| Water Solubility | Soluble in water (10mM) |
| Merck | 14,4219 |
| CAS DataBase Reference | 61718-82-9(CAS DataBase Reference) |
Safety information for Fluvoxamine maleate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Fluvoxamine maleate
| InChIKey | LFMYNZPAVPMEGP-PIDGMYBPSA-N |
| SMILES | C(C1C=CC(=CC=1)/C(=N\OCCN)/CCCCOC)(F)(F)F.C(O)(=O)/C=C\C(O)=O |
Fluvoxamine maleate manufacturer
Lake Chemicals Pvt Ltd
Mehta API Pvt. Ltd.
Humble Healthcare Limited
Ralington Pharma
Cohance Lifesciences (Previously RA Chem Pharma Ltd)
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 61718-82-9 Fluvoxamine maleate 98%View Details
61718-82-9 Fluvoxamine maleate 98%View Details
61718-82-9 -
 Fluvoxamine maleate 98%View Details
Fluvoxamine maleate 98%View Details
61718-82-9 -
 Fluvoxamine Maleate CAS 61718-82-9View Details
Fluvoxamine Maleate CAS 61718-82-9View Details
61718-82-9 -
 61718-82-9 Fluvoxamine maleate 98%View Details
61718-82-9 Fluvoxamine maleate 98%View Details
61718-82-9 -
 Fluvoxamine maleate 99%View Details
Fluvoxamine maleate 99%View Details
61718-82-9 -
 61718-82-9 99%View Details
61718-82-9 99%View Details
61718-82-9 -
 Fluvoxamine maleate 98% CAS 61718-82-9View Details
Fluvoxamine maleate 98% CAS 61718-82-9View Details
61718-82-9 -
 Fluvoxamine maleate CAS 61718-82-9View Details
Fluvoxamine maleate CAS 61718-82-9View Details
61718-82-9