FLUOROACETAMIDE
Synonym(s):NSC 31876
- CAS NO.:640-19-7
- Empirical Formula: C2H4FNO
- Molecular Weight: 77.06
- MDL number: MFCD00008026
- EINECS: 211-363-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
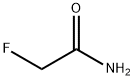
What is FLUOROACETAMIDE?
Description
Fluoroacetamide is an odourless, tasteless, white, crystalline water soluble. It is a fluorinated amide and reacts with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Fluoroacetamide is used as a rodenticide and insecticide to control rabbits but is dangerous for other species of pests, farm livestock, and humans. Fluoroacetamide is a noncombustible substance itself and does not burn but may decompose upon heating to produce irritating, corrosive, and/or toxic fumes. On decomposition, fluoroacetamide releases nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, HF gas, and nitrogen gas.
Chemical properties
crystals
Chemical properties
Fluoroacetamide is a colorless, crystalline solid.
The Uses of FLUOROACETAMIDE
Rodenticide. Insecticide proposed mainly for use on fruits to combat scale insects, aphids, and mites.
What are the applications of Application
Fluoroacetamide is A rodenticide.
Definition
ChEBI: Acetamide substituted at C-2 by a fluorine atom.
Production Methods
Fluoroacetamide is produced by reaction of α-chloroacetamide with potassium fluoride in tetrachloroethylene at elevated temperature.
General Description
Colorless crystalline powder. Used as a rodenticide. Highly toxic.
Air & Water Reactions
Very soluble in water [Farm Chemicals]
Reactivity Profile
FLUOROACETAMIDE is a fluorinated amide. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Hazard
Extremely toxic; poison; mutagen.
Health Hazard
FLUOROACETAMIDE is super toxic; probable oral lethal dose in humans is less than 5 mg/kg, or a taste (less than 7 drops) for a 150-lb. person. Chemically inhibits oxygen metabolism by cells with critical damage occurring to the heart, brain, and lungs resulting in heart failure, respiratory arrest, convulsions, and death.
Fire Hazard
Emits very toxic fumes of fluorine containing compounds and nitrogen oxides when heated to decomposition. Avoid decomposing heat.
Safety Profile
A human poison by an unspecified route. Poison experimentally by ingestion, skin contact, intraperitoneal, subcutaneous, and intravenous routes. Human systemic effects by unspecified route: convulsions, coma, nausea and vomiting. Experimental reproductive effects. Mutation data reported. Used as an insecticide and rodenticide. When heated to decomposition it emits very toxic fumes of Fand NOx. See also FLUORIDES.
Potential Exposure
This material is an organofluorine rodenticide; insecticide proposed mainly for use on fruits to combat scale insects, aphids, and mites. Use is largely restricted to licensed pest control operators.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise fluoroacetamide from chloroform and dry it in a vacuum. [Beilstein 2 IV 454.]
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explo sions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations govern ing storage, transportation, treatment, and waste disposal.
Properties of FLUOROACETAMIDE
| Melting point: | 106-108 °C(lit.) |
| Boiling point: | 259.0±10.0 °C(Predicted) |
| Density | 1.1170 (estimate) |
| solubility | DMSO (Soluble), Methanol (Slightly) |
| form | Solid |
| pka | 14.60±0.40(Predicted) |
| color | Off-White to Pale Beige |
| Merck | 13,4193 |
| BRN | 1739054 |
| Stability: | Stable. |
| CAS DataBase Reference | 640-19-7(CAS DataBase Reference) |
| EPA Substance Registry System | Fluoroacetamide (640-19-7) |
Safety information for FLUOROACETAMIDE
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P262:Do not get in eyes, on skin, or on clothing. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for FLUOROACETAMIDE
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

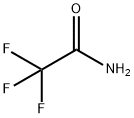
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8