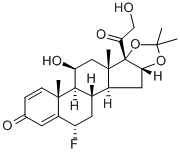
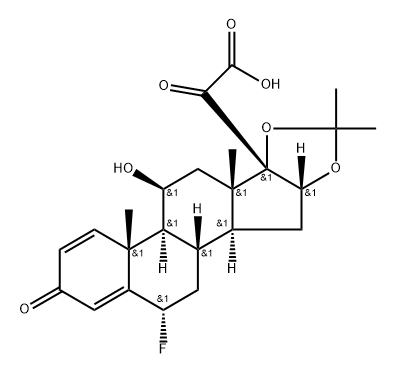
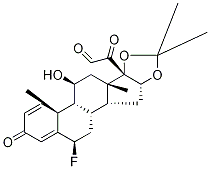
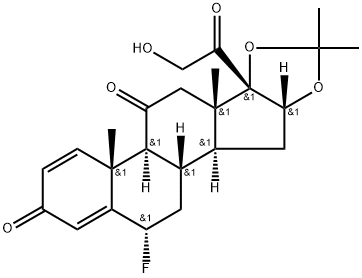
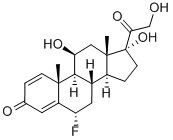
Flunisolide
Synonym(s):6-Fluoro-11,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione 16,17-acetonide
- CAS NO.:3385-03-3
- Empirical Formula: C24H31FO6
- Molecular Weight: 434.5
- MDL number: MFCD00133324
- EINECS: 222-193-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:09
What is Flunisolide?
Absorption
Absorbed rapidly
Description
Flunisolide hemihydrate is administered by inhalation from metered aerosol and used in the management of asthma.
Chemical properties
White Solid
Originator
Syntaris,Syntex,UK,1978
The Uses of Flunisolide
Synthetic fluorinated corticosteroid related to Prednisolone (P703740). Antiasthmatic.
Indications
For the maintenance treatment of asthma as a prophylactic therapy.
Background
Flunisolide (marketed as AeroBid, Nasalide, Nasarel) is a corticosteroid with anti-inflammatory actions. It is often prescribed as treatment for allergic rhinitis and its principle mechanism of action involves activation of glucocorticoid receptors.
What are the applications of Application
Flunisolide-d6 is a fluorinated steroid
Definition
ChEBI: Flunisolide is a fluorinated steroid, a cyclic ketal, a 20-oxo steroid, a 21-hydroxy steroid, an 11beta-hydroxy steroid, a 3-oxo-Delta(1),Delta(4)-steroid and a primary alpha-hydroxy ketone. It has a role as an immunosuppressive agent, an anti-inflammatory drug and an anti-asthmatic drug.
Manufacturing Process
(a) Preparation of 6α-fluoro-16α-hydroxyprednisolone: 1.9 liters of whole
mash containing 400 mg of 6α-fluoroprednisolone (6α-fluoro-11β,17α,21-
trihydroxy-1,4-pregnadiene-3,20-dione) acted upon by Streptomyces
roseochromogenus AE-751 (or Waksman No. 3689) is filtered and the filtrate
extracted three times with 2 liter portions of ethyl acetate. The mycelium is
extracted with 500 ml of ethyl acetate and the mixture filtered. The combined
ethyl acetate extracts are washed with 200 ml of water and concentrated to a
residue. The residue is subjected to partition chromatograph using a 200 g
column of diatomaceous earth moistened with the lower phase of an
equilibrated solvent system composed of 1 volume of water, 5 volumes of
dioxane, and 3 volumes of cyclohexane. The upper phase is used to develop the column and the activity of the eluent is followed by measuring the
ultraviolet absorbance at 240 mμ. The cuts containing most of the activity are
concentrated to a syrupy residue and triturated with acetone. Crystals (25
mg) form and recrystallization gives a product with a MP of 226°C to 230°C.
(b) Preparation of 16α,17α-isopropylidenedioxy-6α-fluoro-1,4-pregnadiene-
11β,21-diol-3,20-dione: 15 mg of crystalline 6α-fluoro-11β,16α,17α,21-
tetrahydroxy-1,4-pregnadiene-3,20-dione [6α-fluoro-16α-hydroxyprednisolone
described in US Patent 2,838,546 and prepared as described in (a) above] is
dissolved in 2 ml of acetone and 0.02 ml of 70% perchloric acid is added. The
solution is allowed to stand 1 hour. Then 0.5 ml of saturated sodium
bicarbonate solution is added and the solution concentrated under reduced
pressure to about 1 ml. The solution is allowed to stand overnight and the
crystals which form are filtered, washed with ether and recrystallized from
acetone-hexane. The crystals are the 16α,17α-isopropylidene derivative of 6α-
fluoro-16α-hydroxyprednisolone.
brand name
Aerobid (Roche); Aerospan Hfa (Forest); Nasalide (IVAX); Nasarel (IVAX).
Therapeutic Function
Antiinflammatory
General Description
The portion of a flunisolide (AeroBid,Nasarel) dose that is swallowed is rapidly converted to the6β-hydroxy metabolite after first-pass metabolism in theliver. The 6β-hydroxy metabolite is approximately as activeas hydrocortisone itself, but the small amount produced usuallyhas limited systemic effects. Water-soluble conjugatesare inactive.
Pharmacokinetics
Flunisolide is a synthetic corticosteroid. It is administered either as an oral metered-dose inhaler for the treatment of asthma or as a nasal spray for treating allergic rhinitis. Corticosteroids are naturally occurring hormones that prevent or suppress inflammation and immune responses. When given as an intranasal spray, flunisolide reduces watery nasal discharge (rhinorrhea), nasal congestion, postnasal drip, sneezing, and itching oat the back of the throat that are common allergic symptoms.
Clinical Use
Flunisolide is an acetone ketal (acetonide) with a 6α-fluoro group and a free C-21 hydroxyl group. The acetonide decreases mineralocorticoid activity, and the 6α-fluoro group increases glucocorticoid activity. It is not a prodrug, because it has the free hydroxyl group at C-21. Flunisolide has approximately 20% of the receptor affinity as budesonide, and approximately 40% of the inhaled dose is systemically bioavailable.
Metabolism
Primarily hepatic, converted to the S beta-OH metabolite.
Metabolism
When administered intranasally or by inhalation, flunisolide is rapidly absorbed from nasal or lung tissue(94). This corticosteroid is efficiently metabolized by the liver to inactive metabolites with no apparent effects on adrenal function with long-term therapy. Flunisolide that is swallowed undergoes extensive first-pass metabolism in the liver, and that which is absorbed directly from the nasopharyngeal mucosa or lung bypasses this initial metabolism. It is not known if the drug undergoes metabolism in the GI tract. Flunisolide is rapidly hydroxylated by CYP3A4 at the 6β position, followed by elimination of the 6α-fluoro group to its more polar 6β-hydroxy metabolite, which attains plasma concentrations that usually are greater than those for flunisolide.
Properties of Flunisolide
| Melting point: | 237-240°C (dec.) |
| Boiling point: | 581.8±50.0 °C(Predicted) |
| Density | 1.1517 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMF: 30 mg/ml; DMSO: 30 mg/ml; Ethanol: 30 mg/ml; Ethanol:PBS (pH 7.2) (1:2): 0.2 mg/ml |
| form | A crystalline solid |
| pka | 12.87±0.10(Predicted) |
| color | White to off-white |
| Merck | 4145 |
| CAS DataBase Reference | 3385-03-3(CAS DataBase Reference) |
Safety information for Flunisolide
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H300:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Flunisolide
Flunisolide manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
You may like
-
 3385-03-3 FLUNISOLIDE 99%View Details
3385-03-3 FLUNISOLIDE 99%View Details
3385-03-3 -
 3385-03-3 98%View Details
3385-03-3 98%View Details
3385-03-3 -
 FLUNISOLIDE CAS 3385-03-3View Details
FLUNISOLIDE CAS 3385-03-3View Details
3385-03-3 -
 Flunisolide CAS 3385-03-3View Details
Flunisolide CAS 3385-03-3View Details
3385-03-3 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0