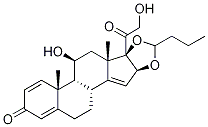
11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
- CAS NO.:13951-70-7
- Empirical Formula: C21H28O6
- Molecular Weight: 376.44
- MDL number: MFCD00872019
- EINECS: 237-731-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
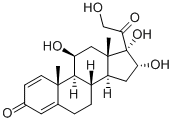
What is 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione?
Chemical properties
White Crystalline Solid
The Uses of 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
A metabolite of Budesonide (B689490), an antiinflammatory agent.
The Uses of 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
11β,16α,17α,21-Tetrahydroxypregna-1,4-diene-3,20-dione (Budesonide EP Impurity A) is a metabolite of Budesonide (B689490), an anti-inflammatory agent.
The Uses of 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
A metabolite of Budesonide, an antiinflammatory agent
What are the applications of Application
11β,16α,17α,21-Tetrahydroxypregna-1,4-diene-3,20-dione is a metabolite of Budesonide
Synthesis
501 g of crude 16α-hydroxy prednisolone acetate is dissolved in 700 ml of dichloromethane and 700 ml of ethanol, then 1.5 ml of formic acid is added, 24 ml of 5% sodium hypochlorite aqueous solution is added dropwise, and the temperature is controlled at 15 °C, and the reaction is stirred to obtain the preliminary processed product; Add 150 ml of 5% sodium bisulfite aqueous solution to the above-mentioned preliminary treatment product, concentrate on removing the solvent, adding water to hydrolyze, and filter to obtain 45 g of 16α-hydroxyprednisolone acetate refined product; 45 g of 16α-hydroxyprednisolone acetate obtained above was dissolved in a mixed organic solvent of 250 ml of dichloromethane and 250 ml of methanol, stirred and cooled to 0 °C, 90 ml of 10% sodium sulfite aqueous solution was added, and the reaction was stirred. After completion, it was neutralized with 15% sulfuric acid by mass concentration; the mixed organic solvent was concentrated, watered out, filtered, and dried to obtain 36.5 g of 16α-hydroxyprednisolone (11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione).
Properties of 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
| Melting point: | 235-238°C |
| Boiling point: | 591.5±50.0 °C(Predicted) |
| Density | 1.38±0.1 g/cm3(Predicted) |
| vapor pressure | 0Pa at 25℃ |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly, Heated) |
| form | Solid |
| pka | 11.93±0.70(Predicted) |
| color | White to Light Yellow |
| Water Solubility | 815mg/L at 20℃ |
| CAS DataBase Reference | 13951-70-7(CAS DataBase Reference) |
Safety information for 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
Computed Descriptors for 11β,16α,17,21-tetrahydroxypregna-1,4-diene-3,20-dione
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde Electrolytic Iron Powder 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 3-(Hydroxymethyl)benzoate N-Boc-2-chloroethylamine 1-Bromo-2-methoxy-3-nitrobenzene N-Methyl-3-cyclopenten-1-amine 2-Bromo-3-hydroxybenzaldehyde 1H-indazole-5-carboxamideRelated products of tetrahydrofuran
![16α,17-[(1RS)-Butylidenebis(oxy)]-11β-hydroxy-17-(hydroxyMethyl)-D-hoMoandrosta-1,4-diene-3,17a-dione
(Mixture of DiastereoMers)](https://img.chemicalbook.in/CAS/GIF/1040085-99-1.gif)



You may like
-
 13951-70-7 11-Beta,16-Alpha,17-Alpha,21-Tetrahydroxypregna-1,4-diene-3,20-dione 99%View Details
13951-70-7 11-Beta,16-Alpha,17-Alpha,21-Tetrahydroxypregna-1,4-diene-3,20-dione 99%View Details
13951-70-7 -
 13951-70-7 98%View Details
13951-70-7 98%View Details
13951-70-7 -
 Budesonide EP Impurity A 98%View Details
Budesonide EP Impurity A 98%View Details
13951-70-7 -
 7441-43-2 98%View Details
7441-43-2 98%View Details
7441-43-2 -
 1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 6-Bromo-3-iodo-1-methyl-1H-indazole 98%View Details
1260741-78-3 -
 4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
4-bromo-3,5-dimethylbenzenesulfonyl chloride 1581266-79-6 98%View Details
1581266-79-6 -
 2490430-37-8 98%View Details
2490430-37-8 98%View Details
2490430-37-8 -
 N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
N-(5-Amino-2-methylphenyl)acetamide 5434-30-0 98%View Details
5434-30-0