Fipronil
- CAS NO.:120068-37-3
- Empirical Formula: C12H4Cl2F6N4OS
- Molecular Weight: 437.15
- MDL number: MFCD00867812
- EINECS: 424-610-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-28 12:01:23
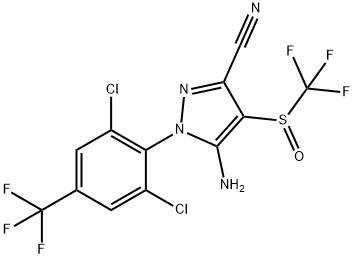
What is Fipronil?
Description
Fipronil is a white powder with a mouldy odour. It has a low solubility in water and is a slow-acting poison. It does not bind strongly with soil, and the half-life of fipronil– sulphone is 34 days. Fipronil is a broadspectrum insecticide of the phenylpyrazole group. Fipronil was first used extensively for the control of ants, beetles, cockroaches, fleas; ticks, termites, mole crickets, thrips, rootworms, weevils, flea of pets, field pest of corn, golf courses, and commercial turf, and other insects. Fipronil was first used in the United States in 1996. Fipronil is a topical insecticide. It kills adult fleas and larvae, ticks, and chewing lice. As a liquid, fipronil is applied on the back side skin of dogs and cats. Reports indicate that some animals do become hypersensitive (allergic) to fipronil.
Description
Fipronil is a broad-spectrum insecticide. It disrupts insects’ central nervous systems by blocking chloride channels that are gated by γ-aminobutyric acid (GABA) or glutamate. These receptors are weaker or nonexistent in mammals.
Fipronil has a chiral center at the sulfur atom in the sulfoxide group; the two enantiomers have been isolated and verified. The article of commerce is a racemic mixture of the two.
Fipronil formulations sold worldwide include flea-control products for pets, cockroach traps, and insecticides for agrciultural crops, lawns, golf courses, and the like. But despite its wide range of uses and relative harmlessness toward mammals, fipronil is a significant environmental hazard. It is highly toxic to fish and other waterborne wildlife, birds, and beneficial insects. It has been cited as one of the primary causes of honeybee die-off.
In 2017, fipronil was the culprit in a food-contamination scandal in the Netherlands. Molecule of the Week’s Dutch reader who suggested this molecule provided this narrative:
A recent article in the Dutch newspaper De Stentor chronicles the fipronil scandal, including the subsequent legal fallout and a better way to combat red mites. (Note: The article is in Dutch, but it is easily translated by major Web browsers.)
Chemical properties
White SOlid
Chemical properties
White crystalline solid.
The Uses of Fipronil
Fipronil is used for the control of a wide range of insect species in rice, cereals, corn, cotton, top fruit, sugar beet, sugar cane, oilseed rape, many vegetables and other high-value crops. It also has a veterinary use as an ectoparasiticide.
The Uses of Fipronil
A broad spectrum insecticide which actively disrupts the CNS of contacted insects by blocking the passage of chloride ions through GABA receptors.
The Uses of Fipronil
antifungal
The Uses of Fipronil
Pesticide.
What are the applications of Application
Fipronil is a GluCl and GABA-gated-Cl channel blocker
Definition
ChEBI: 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-4-[(trifluoromethyl)sulfinyl]-1H-pyrazole-3-carbonitrile is a member of the class of pyrazoles that is 1H-pyrazole that is substituted at positions 1, 3, 4, and 5 by 2,6-dichloro-4-(trifluoromethyl)phenyl, cyano, (trifluoromethyl)sulfinyl, and amino groups, respectively. It is a nitrile, a dichlorobenzene, a primary amino compound, a member of pyrazoles, a sulfoxide and a member of (trifluoromethyl)benzenes.
Agricultural Uses
Insecticide, Veterinary medicine: Not approved for use in EU countries. Fipronil was introduced into the U.S. in 1996 for use in animal health and indoor pest control. It is the constituent of many products for controlling a wide spectrum of domestic animal and residential pests.
Trade name
BES® 602; CEASEFIRE®; CHIPCO®; COMBAT®; FRONTLINE; MB-46030®; H&G®; ICON®; MAXFORCE® ANT STATION; MAXFORCE® ROACH STATION; REGENCY SOFION®; REGENT®; REGENT® 500-FS; TERMIDOR® L VI-NIL
Pharmacology
Fipronil is a phenylpyrazole, the mode of action of which
is to inhibit nerve transmission in arthropods by blocking
γ -aminobutyric acid-gated chloride channels. Fipronil is
available as spray and spot-on formulations to control
fleas and ticks on cats and dogs. The adulticidal activity of
fipronil accounts for the majority of its activity, although
additional activity against flea eggs and larvae results
from the presence of fipronil on hairs and debris shed into
the environment from treated pets.
Autohistoradiography studies (11) into the cutaneous
distribution of 14C-fipronil in the cat and dog following
spot-on administration demonstrated that radioactivity
was restricted principally to the stratum corneum, the
viable epidermis, and the pilosebaceous units. Following
its slow release from sebaceous glands, fipronil migrates in
the sebum covering the skin and hairs by passive diffusion
and was shown to persist on hair for up to 2 months after
treatment.
Potential Exposure
Pyrazole/phenylpyrazole/organofluor ine insecticide; veterinary medicine. Fipronil was intro duced into the United States in 1996 for use in animal health and indoor pest control. It is the constituent of many products for controlling a wide spectrum of domestic ani mal and residential pests. Banned for use in EU.
Metabolic pathway
Through the abiotic degradation of fipronil in aqueous solution and on the soil surface, 5-amino-3- carbamoyl-1-[2,6-dichloro-4-(trifluoromethyl)-phenyl]-4- [(trifluoromethyl)sulfinyl]pyrazole is the only hydrolysis product detected. Fipronil in acidic aqueous solution exposed under a xenon lamp degrades with the concomitant appearance of 5-amino-3-cyano-1-[2,6- dichloro-4-(trifluoromethyl)-phenyl]-4- (trifluoromethyl)pyrazole and 5-amino-3-cyano-1-[2,6- dichloro-4-(trifluoromethyl)phenyl]pyrazole-4-sulfonic acid. Under field conditions, when fipronil is applied in formulation, four metabolites which include one product resulting from reduction on the sulfur atom of fipronil are detected.
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN2902 Pesticide, liquid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Degradation
Fipronil is stable in water at pH 5 and 7 but is slowly hydrolysed at pH 9 (DT50 28 days) to the amide (2). It undergoes rapid aqueous photolysis with a DT50 of ﹤0.5 day. The major photodecomposition product in water and on soil surfaces is desulfinyl-fipronil(3) which is formed by extrusion of the SO group. A minor photoproduct in water, on soil and on plant surfaces is the sulfonic acid (4). The mechanisms of these reactions are discussed in a recent paper by Bobe et al. (1998).
Toxicity evaluation
Acute oral LD50 for rats: 100 mg/kg
Waste Disposal
It is the responsibility of chemical waste generators to determine the toxicity and physical properties and of a discarded chemical and to properly identify its classification and certification as a haz ardous waste and to determine the disposal method. United States Environmental Protection Agency guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult and fol low all regional, national, state and local hazardous waste laws to ensure complete and accurate classification and dis posal methods.
Properties of Fipronil
| Melting point: | 200-201°C |
| Boiling point: | 510.1±50.0 °C(Predicted) |
| Density | 1.477-1.626 |
| vapor pressure | 3.7×10-7 Pa (25 °C) |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | DMSO: 250 mg/mL (571.89 mM) |
| appearance | white to yellow powder |
| form | neat |
| Water Solubility | 1.9-2.4 mg 1l-1 (20 °C) |
| pka | -5.86±0.20(Predicted) |
| form | Solid |
| color | White to Light yellow |
| λmax | 208nm(H2O)(lit.) |
| Merck | 14,4085 |
| BRN | 8090115 |
| CAS DataBase Reference | 120068-37-3(CAS DataBase Reference) |
| EPA Substance Registry System | Fipronil (120068-37-3) |
Safety information for Fipronil
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H372:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P314:Get medical advice/attention if you feel unwell. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Fipronil
Fipronil manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran




You may like
-
 Fipronil 98%View Details
Fipronil 98%View Details
120068-37-3 -
 120068-37-3 Fipronil 99%View Details
120068-37-3 Fipronil 99%View Details
120068-37-3 -
 Fipronil 97% CAS 120068-37-3View Details
Fipronil 97% CAS 120068-37-3View Details
120068-37-3 -
 Fipronil 98%View Details
Fipronil 98%View Details
120068-37-3 -
 Fipronil CAS 120068-37-3View Details
Fipronil CAS 120068-37-3View Details
120068-37-3 -
 Fipronil 120068-37-3 99%View Details
Fipronil 120068-37-3 99%View Details
120068-37-3 -
 Fipronil CAS 120068-37-3View Details
Fipronil CAS 120068-37-3View Details
120068-37-3 -
 Fipronil CAS 120068-37-3View Details
Fipronil CAS 120068-37-3View Details
120068-37-3
