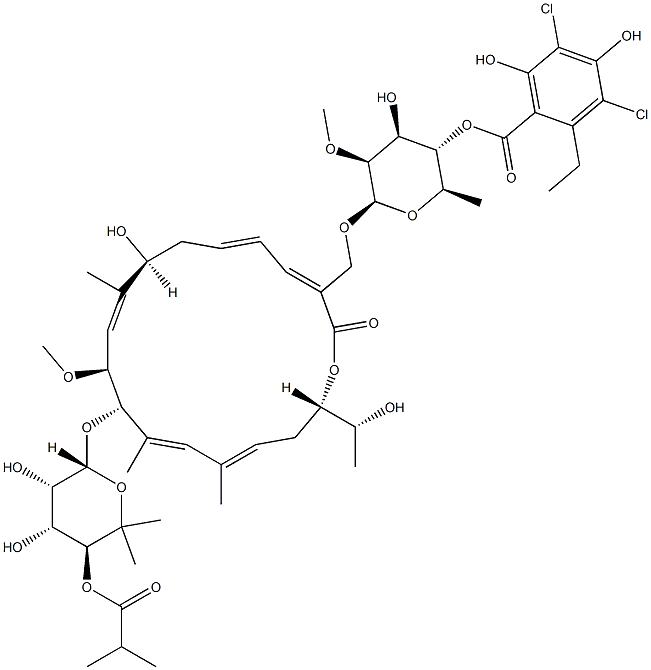
FidaxoMicin
Synonym(s):Clostomicin B1;Lipiarmycin A3;OPT 80;Tiacumicin B
- CAS NO.:873857-62-6
- Empirical Formula: C52H74Cl2O18
- Molecular Weight: 1058.04
- MDL number: MFCD27976367
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is FidaxoMicin?
Absorption
Following oral administration of a single dose of 200 mg fidaxomicin in healthy adults, the Cmax of fidaxomicin and its main metabolite OP-1118 were 5.20 ± 2.81 ng/mL and 12.0 ± 6.06, respectively. The median Tmax of fidaxomicin was 2 hours. The systemic absorption of fidaxomicin following oral administration is minimal.
In a food-effect study involving healthy adults in either with a high-fat meal versus under fasting conditions, the Cmax of fidaxomicin and OP-1118 were decreased by 21.5% and 33.4%, respectively; however, this effect is deemed to be clinically insignificant as the therapeutic action of fidaxomicin does not depend on drug concentrations in the systemic circulation.
Toxicity
In rats, the LD50 of fidaxomicin was approximately 200 mg/kg and the no observed adverse effect level (NOAEL) was determined to be 62.5 mg/kg following administration of a single intravenous dose.
There is limited clinical data on acute overdose in humans.
Description
Fidaxomicin (OPT-80) was approved by the U.S. FDA in May 2011 for
the treatment of Clostridium difficile-associated diarrhea (CDAD), joining
metronidazole and vancomycin as drugs recommended for treatment of
C. difficile infections (CDI). Fidaxomicin, also known as
lipiarmycin and tiacumicin, is an 18-membered macrolide natural
product that was first reported in mid-1970s and is produced by
fermentation. Fidaxomicin and its primary metabolite OP-1118,
which results from hydrolysis of the isobutyryl ester, are narrowspectrum
antibacterial agents with activity against gram-positive aerobic
and anaerobic organisms, but not against gram-negative organisms.
Fidaxomicin and OP-1118 exert their antibacterial activity by inhibiting
bacterial RNA polymerase, thereby inhibiting bacterial protein
synthesis.
The MIC90 (minimum inhibitory concentration to kill 90% of
bacteria) for fidaxomicin against C.difficile is 0.125–0.25 μg/mL; OP-
1118 is 4- to 16-fold less potent than the parent compound.
Fidaxomicin has been reported to spare native intestinal flora such as
Bacteroides spp. and as such, may prevent selection of drug-resistant
bacteria. Fidaxomicin is bactericidal to C. difficile and has a low
propensity for resistance development with no cross-resistance to
existing antibiotics. Fidaxomicin shows minimal systemic absorption following oral administration in preclinical studies and humans.
Originator
Optimer Pharmaceuticals (United States)
The Uses of FidaxoMicin
Fidaxomicin is a recently marketed antibiotic with a confusing history dating back to its original isolation in 1975. Fidaxomicin is the major analogue of a family of macrocyclic lactones, isolated independently by three different groups from cultures belonging to three different genera (Actinoplanes, Dactylosporangium and Micromonospora) known as lipiarmycin A3, tiacumicin B and clostomicin B1, respectively. Fidaxomicin is a narrow spectrum antibiotic with excellent activity against Gram positive bacteria, notably Clostridium difficile. Fidaxomicin acts in the gastrointestinal tract without undue disruption to gut microbial flora.
The Uses of FidaxoMicin
Fidaxomycin is a natural macrocyclic antibiotic that inhibits RNA polymerase with selectivity for Gram-positive bacteria over Gram-negative bacteria (IC50s = 0.4 and 6 μM, respectively). It has potent antibacterial activity against most Gram-positive bacteria and effectively targets the Gram-positive C. difficile (MIC = 12 ng/ml). Orally administered fidaxomycin exhibits minimal systemic bioavailability resulting in maximal gastrointestinal tract distribution. Fidaxomycin is effective in clearing C. difficile infections while sparing Gram-negative bacteria in the gut.[Cayman Chemical]
The Uses of FidaxoMicin
Fidaxomicin is a nonabsorbed macrocyclic antibiotic, and is the first antimicrobial to be approved by the FDA for the treatment of Clostridium difficile infection (CDI) in 20 years. Fidaxomicin works by inhibiting sporulation by CDI, sustaining clinical response and reducing recurrences of this pathogen.
Indications
Fidaxomicin is indicated for the treatment of Clostridioides (formerly Clostridium) difficile-associated diarrhea in adult and pediatric patients 6 months of age and older.
Fidaxomicin should only be used in patients with proven or strongly suspected C. difficile infection to reduce the risk of development of drug-resistant bacteria and maximize the therapeutic effectiveness of fidaxomicin and other antimicrobial agents.
Background
Fidaxomicin is a novel macrolide antibiotic used in the treatment of diarrhea caused by Clostridioides (formerly Clostridium) difficile in adult and pediatric patients over the age of 6 months. Fidaxomicin is a naturally-occurring 18-member macrocycle derived from fermentation. Because fidaxomicin contains an 18-membered lactone ring in its structure, it is referred to as a macrocyclic lactone antibiotic drug. The antibacterial activity of fidaxomicin is distinct from macrolides and rifamycins, as the bactericidal activity is time-dependent, and not concentration-dependent. Fidaxomicin was the first macrocyclic lactone antibiotic with activity against C. difficile, and it displays a narrow spectrum of activity against gram-positive anaerobes. It mediates its potent bactericidal action on the bacteria by inhibiting the bacterial RNA synthase, thereby disrupting bacterial transcription. The minimum inhibitory concentration (MIC90) for fidaxomicin is four times less than that of vancomycin, which was the primary drug of choice for C. difficile infection before the approval of fidaxomicin. Unlike vancomycin, however, fidaxomicin has a negligible effect on normal colonic microflora.
The FDA initially approved fidaxomicin in May 2011 for the treatment of C. difficile-associated diarrhea in adult patients over the age of 18. Later that year in December, the drug was also approved by the European Medicine Agency. In June 2012, fidaxomicin was also granted approval by Health Canada. The approved indication of fidaxomicin was expanded by the FDA in January 2020 to include pediatric patients over the age of 6 months in the treatment population.
What are the applications of Application
Fidaxomicin is a macrocyclic antibiotic
Definition
ChEBI: An 18-membered macrolide that is a fermentation product obtained from the Actinomycete Dactylosporangium aurantiacum. A narrow spectrum antibiotic used for treatment of Clostridium difficile-related infections.
brand name
Dificid
Pharmaceutical Applications
Formerly known as difimicin. An 18-membered macrocyclic compound related to the tiacumicin group of antibiotics rather than conventional macrolides. It is active against staphylococci (MIC 0.5–2 mg/L) and most anaerobic Grampositive bacilli and cocci, but Gram-negative bacilli, including Gram-negative anaerobes, are resistant. It is very poorly absorbed when given orally and most interest surrounds its activity against C. difficile (MIC 0.12–0.25 mg/L). Such data as are presently available from clinical trials suggest that it is as safe and effective in the treatment of C. difficile-associated diarrhea as vancomycin.
Biochem/physiol Actions
Fidaxomicin is a first-in-class macrocyclic antibacterial agent for gram positive bacteria treatment, notably Clostridium difficile infections. Fidaxomicin produces its antibacterial effects by inhibiting bacterial RNA polymerase at transcription initiation. Furthermore, Fidaxomicin is an inhibitor of bacterial transcription. Fidaxomicin acts at an earlier step in the transcription initiation pathway. Specifically, Fidaxomicin binds to the DNA template-RNA polymerase complex and prevents the initial separation of DNA strands, which precedes messenger RNA synthesis by inhibiting the s subunit. Fidaxomicin′s unique target site may explain its limited spectrum of antimicrobial activity because s subunits differ among bacterial species.
Pharmacokinetics
Fidaxomicin has a narrow-spectrum antibacterial profile, with potent bactericidal activity specifically against C. difficile. The minimum inhibitory concentration for 90% of organisms for fidaxomicin against C. difficile ranged from 0.0078 to 2 μg/mL in vitro. The bactericidal activity of fidaxomicin is time-dependent. Other than C. difficile, fidaxomicin has moderate inhibitory activity against Gram-positive bacteria (S. aureus and Enterococcus spp.) and poor activity against normal colonic flora, including anaerobes and enteric Gram-negative bacilli. Isolates of C. difficile that are resistant to rifamycins or other antimicrobial classes (such as cephalosporins, fluoroquinolones, clindamycin) were not shown to be cross-resistant to fidaxomicin.
Clinical Use
Macrolide antibacterial agentTreatment of Clostridium Difficile infection
Drug interactions
Potentially hazardous interactions with other drugs Anti-arryhthmics: avoid concomitant use with amiodarone and dronedarone. Antibaterials: avoid concomitant use with clarithromycin and erythromycin. Antifungals: avoid concomitant use with ketoconazole. Calcium channel blockers: avoid concomitant use with verapamil. Ciclosporin: increased fidaxomicin levels, avoid concomitant use.
Metabolism
Following oral administration, fidaxomicin is transformed to its main and pharmacologically active metabolite, OP-1118, via hydrolysis at the isobutyryl ester. As cytochrome enzymes are not involved in the metabolism of fidaxomicin, it is speculated that this biotransformation is mediated by gastric acid or enzymatic activity of intestinal microsomes.
Metabolism
Mainly metabolised by hydrolysis in the gut at the isobutyryl ester to form its main and microbiologically active metabolite, OP-1118. Over 92% of a dose is excreted in the faeces as either fidaxomicin or OP-1118, although very small amounts of OP-1118 have been recovered in the urine
Properties of FidaxoMicin
| Melting point: | 161 °C |
| Boiling point: | 1046.4±65.0 °C(Predicted) |
| Density | 1.33 |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly, Heated), DMSO (Slightly, Heated), Methanol (Slightly) |
| form | powder |
| pka | 5.09±0.35(Predicted) |
| color | White to Off-White |
Safety information for FidaxoMicin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P321:Specific treatment (see … on this label). P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for FidaxoMicin
FidaxoMicin manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![4-Chloro-N-[2-[[5-(trifluoromethyl)-2-pyridinyl]sulfonyl]ethyl]benzamide](https://img.chemicalbook.in/CAS/GIF/188591-46-0.gif)
You may like
-
 873857-62-6 Fidaxomicin 98%View Details
873857-62-6 Fidaxomicin 98%View Details
873857-62-6 -
 Fidaxomicin CAS 873857-62-6View Details
Fidaxomicin CAS 873857-62-6View Details
873857-62-6 -
 Fidaxomicin CAS 873857-62-6View Details
Fidaxomicin CAS 873857-62-6View Details
873857-62-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1