Ferric carboxymaltose
- CAS NO.:9007-72-1
- Empirical Formula: C39H63FeO39
- Molecular Weight: 1211.73912
- EINECS: 813-933-0
- Update Date: 2025-05-15 12:11:39
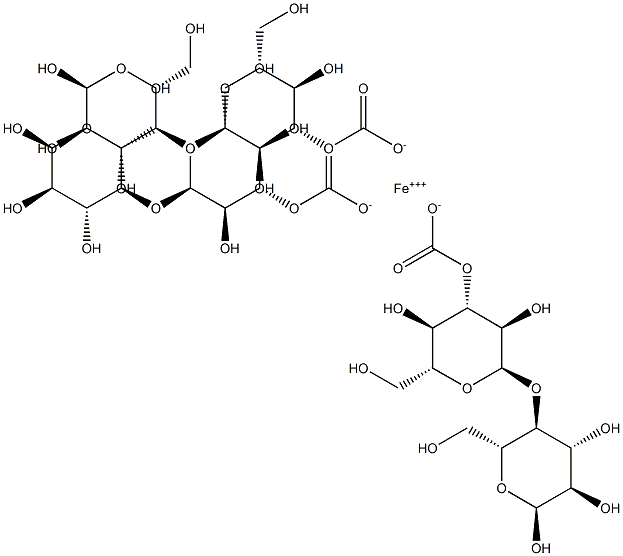
What is Ferric carboxymaltose?
Absorption
When a single dose of 100 to 1000 mg of iron was given to iron deficient patients, the maximum serum concentration (Cmax) was 37 μg/mL to 333 μg/mL. These levels were obtained 15 minutes to 1.21 hours post dose (Tmax).
Toxicity
The most common adverse reactions (>2%) are nausea, hypertension, flushing, hypophosphatemia, and dizziness.
Background
Ferric Carboxymaltose is an iron replacement product and chemically, an iron carbohydrate complex. FDA approved on July 25, 2013.
Indications
Ferric carboxymaltose is an iron replacement product indicated for the treatment of iron deficiency anemia in patients ≥1 year of age who have an intolerance to, or unsatisfactory response from, oral iron therapy. It is also indicated in adult patients who have non-dialysis-dependent chronic kidney disease.
Mechanism of action
Ferric carboxymaltose is a macromolecular ferric hydroxide carbohydrate complex, which allows for controlled delivery of iron within the cells of the reticuloendothelial system and subsequent delivery to the iron-binding proteins ferritin and transferrin, with minimal risk of release of large amounts of ionic iron in the serum. Intravenous administration of ferric carboxymaltose results in transient elevations in serum iron, serum ferritin and transferrin saturation, and, ultimately, in the correction of haemoglobin levels and replenishment of depleted iron stores.
Pharmacokinetics
When measured using positron emission tomography (PET), the red cell uptake of 59-Fe and 52-Fe from INJECTAFER ranged from 61% to 99%. In patients with iron deficiency, the red cell uptake ranged from 91% to 99%. In patients with renal anemia, the red cell uptake ranged from 61% to 84%.
Clinical Use
Ferric carboxymaltose complex:Treatment of iron deficiency anaemia (when oral treatment is ineffective or contraindicated)
Side Effects
Ferric carboxymaltose was well tolerated, with most drug-related adverse events being mild to moderate in severity. Commonly reported drug-related adverse events included headache, dizziness, nausea, abdominal pain, constipation, diarrhoea, rash and injection site reactions.
The incidence of drug-related adverse events in patients receiving intravenous ferric carboxymaltose was generally similar to that in patients receiving oral ferrous sulfate. In general, rash and local injection-site reactions were more common with ferric carboxymaltose, whereas gastrointestinal adverse events were more frequent with ferrous sulfate. In patients with chronic kidney disease undergoing haemodialysis, a lower proportion of ferric carboxymaltose than iron sucrose recipients experienced at least one drug-related adverse event.
Drug interactions
Potentially hazardous interactions with other drugs Dimercaprol: avoid concomitant use. Oral iron: reduced absorption
Metabolism
Not Available
Metabolism
Most absorbed iron is bound to transferrin and transported to the bone marrow where it is incorporated into haemoglobin; the remainder is contained within the storage forms, ferritin or haemosiderin, or as myoglobin, with smaller amounts occurring in haem-containing enzymes or in plasma bound to transferrin. Only very small amounts of iron are excreted as the majority released after the destruction of the haemoglobin molecule is re-used.
Properties of Ferric carboxymaltose
Safety information for Ferric carboxymaltose
Computed Descriptors for Ferric carboxymaltose
| InChIKey | CRTIFGUDJMSNSV-SJEGEKMXNA-K |
Ferric carboxymaltose manufacturer
Sumar biotech LLP
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 9007-72-1 Ferric Carboxymaltose 98%View Details
9007-72-1 Ferric Carboxymaltose 98%View Details
9007-72-1 -
 9007-72-1 98%View Details
9007-72-1 98%View Details
9007-72-1 -
 Ferric Carboxymaltose 9007-72-1 99%View Details
Ferric Carboxymaltose 9007-72-1 99%View Details
9007-72-1 -
 9007-72-1 Ferric Carboxymaltose 98%View Details
9007-72-1 Ferric Carboxymaltose 98%View Details
9007-72-1 -
 9007-72-1 98%View Details
9007-72-1 98%View Details
9007-72-1 -
 Ferric Carboxymaltose 98%View Details
Ferric Carboxymaltose 98%View Details
9007-72-1 -
 Ferric carboxy maltose 9007-72-1 98%View Details
Ferric carboxy maltose 9007-72-1 98%View Details
9007-72-1 -
 Ferric Carboxymaltose (Encicarb 500mg/10ml)View Details
Ferric Carboxymaltose (Encicarb 500mg/10ml)View Details
9007-72-1
