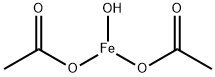
FERRIC ACETATE
- CAS NO.:10450-55-2
- Empirical Formula: C4H7FeO5
- Molecular Weight: 190.94
- MDL number: MFCD00054407
- EINECS: 233-936-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:55
What is FERRIC ACETATE?
Description
Ferric acetate is the coordination compound more commonly known as "basic iron acetate". With the formula [Fe3O(OAc)6(H2O)3]OAc (OAc is CH3CO2-), it is a salt, composed of the cation [Fe3(μ3-O)(OAc)6(H2O)3]+ and an acetate anion . The formation of the red-brown complex has long been used as a test for ferric ions.
Chemical properties
Red powder. Soluble in alcohol and acids; insoluble in water. Combustible.
Physical properties
Basic iron acetate forms on treating aqueous solutions of iron (III) sources with acetate salts.
Early work showed that it is trinuclear.The Fe centres are equivalent, each being octahedral, being bound to six oxygen ligands, including a triply bridging oxide at the center of the equilateral triangle.The compound was an early example of a molecular compound of iron that features an oxide ligand. Ignoring its 24 hydrogen centres, the cation has D3h symmetry.
The Uses of FERRIC ACETATE
In the textile industry as a mordant in dyeing and printing, and for the weighting of silk and felt; as wood preservative; in leather dyes.
The Uses of FERRIC ACETATE
Materials prepared by heating iron, acetic acid, and air, loosely described as basic iron acetates, are used as dyes and mordants.
Reactions
The terminal aqua ligands on the trimetallic frame work can be substituted with other ligands, such as pyridine and dimethyl formamide . Many different salts are known by exchanging the anion, e.g. [Fe3(μ3-O)(OAc)6(H2O)3]Cl. Reduction of the cation affords the neutral mixed-valence derivative that contains one ferrous and two ferric centers. Mixed metal species are known such as [Fe2CoO(OAc)6(H2O)3].
Properties of FERRIC ACETATE
| solubility | insoluble in H2O; soluble in ethanol, acid solutions |
| form | brown-red amorphous powder |
| color | brown-red amorphous
powder |
| Water Solubility | insoluble H2O; soluble EtOH, acid [CRC10] |
| CAS DataBase Reference | 10450-55-2 |
Safety information for FERRIC ACETATE
Computed Descriptors for FERRIC ACETATE
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Ferric acetate CAS 10450-55-2View Details
Ferric acetate CAS 10450-55-2View Details
10450-55-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8