Fenticonazole nitrate
- CAS NO.:73151-29-8
- Empirical Formula: C24H21Cl2N3O4S
- Molecular Weight: 518.41
- MDL number: MFCD00941391
- EINECS: 277-302-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-11-22 16:52:42
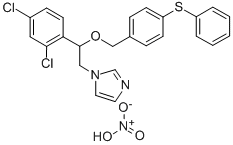
What is Fenticonazole nitrate?
Description
Fenticonazole nitrate is an antifungal imidazole derivative reported to be more effective than other imidazole antifungals such as miconazole, clotrimazole and econazole. In addition to its potent, broad-spectrum fungicidal activity, fenticonazole is also active against some Gram-positive bacteria.
Description
Fenticonazole is an imidazole with antimicrobial activity. It is active against clinical isolates of Candida species, including C. albicans, C. parapsilosis, C. tropicalis, C. krusei, and C. guilliermondii with MIC values ranging from 0.12 to 32 μg/ml. It is also active against clinical isolates of bacteria, including B. fragilis, associated with bacterial vaginosis (MICs = ≤0.03-16 μg/ml), as well as bacteria, including S. aureus and S. epidermidis, associated with skin infections with MIC values ranging from 0.03 μg/ml or less to greater than 16 μg/ml. Fenticonazole inhibits fungal growth in a guinea pig model of experimental M. canis dermatomycosis (ED50 = 0.5%, applied topically).
Chemical properties
Off-White Solid
Originator
Recordati (Italy)
The Uses of Fenticonazole nitrate
Broad spectrum antimycotic; also active as antibacterial. Antifungal (topical).
What are the applications of Application
Fenticonazole Nitrate is an azole antifungal drug that inhibits CYP51A1
Definition
ChEBI: A racemate comprising equimolar amounts of (R)- and (S)-fenticonazole nitrate. Used for the treatment of vaginal candidiasis.
Manufacturing Process
1-(2',4'-Dichlorophenyl)-2-chloroethanol:
49.5 g of sodium borohydride were added slowly and in small parts to a
suspension of 233 g of 1-(1'-hydroxy-2'-chloroethyl)-2,4-dichlorobenzene in 1
liter of methanol stirred at room temperature. The solution thus obtained was
stirred at room temperature for a further two hours, and it was then poured
into 1 liter of 5 N hydrochloric acid cooled with ice. After extraction with ethyl
acetate or chloroform, the extract was washed with water, with 1 N sodium
hydroxide, then again with water until neutrality, and finally with a saturated
sodium chloride solution. The extract was dried, the solvent evaporated off
and 220 g of an oil were obtained. The oil solidified on standing and the solid
1-(2',4'-dichlorophenyl)-2-chloro-ethanol melted at 48-51°C.
1-(2',4'-Dichlorophenyl)-2-(N-imidazolyl)ethanol:
30 g of sodium were added to a solution of 88.5 g of imidazole in 600 ml of
methanol; the solvent was then evaporated off. The residue was dissolved in
300 ml of dimethylformamide and heated to 115-120°C. To the solution so
obtained was added, dropwise and under stirring, a solution of 225 g of 1-
(2',4'-dichlorophenyl)-2-chloroethanol in 400 ml of dimethylformamide. The
mixture was heated to 115-120°C and maintained at that temperature for 20
min and, after subsequent cooling to 40°C, 2500 ml of iced water were added
under vigorous stirring. The product precipitated under stirring over a period
of about two hours, the upper liquid was then decanted off, a further 2500 ml
of water were added and, after standing, the whole was filtered. The
precipitate thus obtained was dried and crystallized from toluene. 170 g of the
1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)ethanol, melting at 134-135°C, was
obtained.
METHOD 1:
A solution of 2.57 g of 1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)ethanol in 10
ml of hexamethylphosphoramide was dropped at 25°C into a suspension of
0.52 g of sodium hydride (50% in oil) in 5 ml of hexamethylphosphoramide.
When hydrogen emission was over, the salification was completed by heating
for 1 hour at 50°C. After cooling to 25°C, 2.58 g of 1-chloromethyl-4-
phenylthiobenzene were added. The temperature was raised to 50°C and
maintained at that temperature for 12 hours. At the end of the reaction, the
mixture was poured into 200 ml of water, the product was extracted with
diethyl ether, the solvent was evaporated off and the residue was purified
twice on a silica gel column, using ethyl acetate as eluant and testing the
various fractions by TLC. The solvent was evaporated off the middle fractions to give 2.4 g of the 1-[2,4-dichloro-beta-[[p-(phenylthio)benzyl]oxy]
phenethyl]imidazole as a yellowish oil, showing a single spot on TLC.
METHOD 2:
0.66 g of sodium hydride (50% in oil) were added at 20-30°C and under
nitrogen atmosphere to 3.86 g of 1-(2',4'-dichlorophenyl)-2-(N-imidazolyl)
ethanol in 15 ml of dimethylsulphoxide (dried on calcium hydride). The
mixture was heated under stirring at 50-60°C until gas emission was over.
After cooling to 20-25°C, 0.5 g of potassium iodide were added and slowly a
solution of 3.51 g of 1-chloromethyl-4-phenylthiobenzene in 4 ml of
dimethylsulphoxide was dropped in. The mixture was stirred at 20-25°C until
addition of the 1-chloromethyl-4-phenylthiobenzene was over. The mixture
was then poured into 150 ml of water and extracted with diethyl ether. To the
etheric solution, after drying on anhydrous sodium sulphate, was added
excess 4 N nitric acid solution in diethyl ether: the desired product
precipitated as nitrate, an oil which solidified on standing. After standing for
20 hours, the etheric liquid was decanted off and the residue was crystallized
from ethanol. The nitrate thus obtained, not completely pure, was dissolved in
water and excess sodium carbonate was added in order to liberate the base
which was then extracted with ethyl acetate. The base, obtained by filtration,
was purified on a silica gel column using ethyl acetate as eluant. The
combined fractions containing the desired product were evaporated to
dryness. The residue was dissolved in diethyl ether, again transformed into
the nitrate and crystallized from ethanol. Yield: 3.1 g of a white crystalline
powder, melting at 136°C; λmax 252 nm (methanol).
brand name
Lomexin
Therapeutic Function
Antifungal
Properties of Fenticonazole nitrate
| Melting point: | 135-137°C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Practically insoluble in water, freely soluble in dimethylformamide and in methanol, sparingly soluble in anhydrous ethanol |
| form | neat |
| pka | 6.54(at 25℃) |
| form | Solid |
| color | White to Pale Yellow |
| Merck | 14,4005 |
| CAS DataBase Reference | 73151-29-8(CAS DataBase Reference) |
Safety information for Fenticonazole nitrate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Fenticonazole nitrate
| InChIKey | FJNRUWDGCVDXLU-UHFFFAOYSA-N |
Fenticonazole nitrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Fenticonazole nitrate 99%View Details
Fenticonazole nitrate 99%View Details -
 Fenticonazole nitrate 98%View Details
Fenticonazole nitrate 98%View Details -
 Fenticonazole nitrate 99%View Details
Fenticonazole nitrate 99%View Details
73151-29-8 -
 Fenticonazole nitrate 73151-29-8 99%View Details
Fenticonazole nitrate 73151-29-8 99%View Details
73151-29-8 -
 Fenticonazole Nitrate CAS 73151-29-8View Details
Fenticonazole Nitrate CAS 73151-29-8View Details
73151-29-8 -
 Fenticonazole nitrate 98% (HPLC) CAS 73151-29-8View Details
Fenticonazole nitrate 98% (HPLC) CAS 73151-29-8View Details
73151-29-8 -
 Fenticonazole nitrate CAS 73151-29-8View Details
Fenticonazole nitrate CAS 73151-29-8View Details
73151-29-8 -
 Fenticonazole Nitrate API MANUFACTURER INDIAView Details
Fenticonazole Nitrate API MANUFACTURER INDIAView Details
73151-29-8
