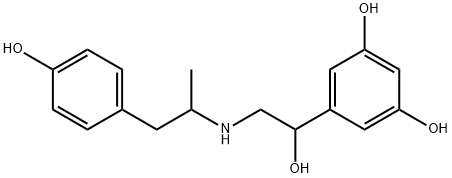
FENOTEROL HYDROBROMIDE
Synonym(s):2-(3,5-Dihydroxyphenyl)-2-hydroxy-2′-(4-hydroxyphenyl)-1′-methyldiethylamine hydrobromide
- CAS NO.:1944-12-3
- Empirical Formula: C17H21NO4.BrH
- Molecular Weight: 384.27
- MDL number: MFCD00079288
- EINECS: 217-742-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
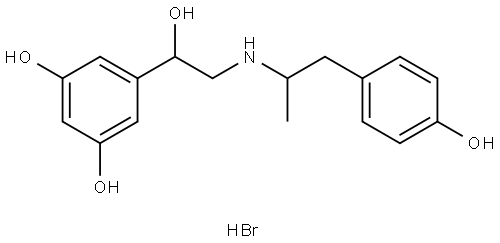
What is FENOTEROL HYDROBROMIDE?
Chemical properties
White Solid
Originator
Berotec,Boehringer Ingelheim,W. Germany,1972
The Uses of FENOTEROL HYDROBROMIDE
An β2-adrenergic agonist. Bronchodilator; tocolytic.
What are the applications of Application
Fenoterol hydrobromide is a β2-adrenergic activator
Definition
ChEBI: The hydrobromide salt of fenoterol. A beta2-adrenergic agonist, it is used as a bronchodilator in the management of reversible airway obstruction.
Manufacturing Process
441 grams (1.4 mols) of 3,5-diacetoxy-α-bromo-acetophenone (MP 66°C),
prepared by bromination of 3,5-diacetoxy-acetophenone, were added to a
solution of 714 grams (2.8 mols) of 1-p-methoxyphenyl-2-benzylaminopropane
in 1,000 cc of benzene, and the resulting solution mixture was
refluxed for 1 hour. The molar excess of 1-p-methoxy-phenyl-2-benzylaminopropane
precipitated out as its hydrobromide. After separation of the
precipitated hydrobromide of the amino component, the hydrochloride of 1-pmethoxy-
phenyl-2-(β-3',5'-diacetoxyphenyl-β-oxo)-ethyl-benzylamino-propane
was precipitated from the reaction solution by addition of an ethanolic solution
of hydrochloric acid. The precipitate was separated and, without further
purification, was deacetylated by boiling it in a mixture of 2 liters of aqueous
10% hydrochloric acid and 1.5 liters of methanol.
The resulting solution was filtered through animal charcoal and, after addition
of 2 liters of methanol, it was debenzylated by hydrogenation at 60°C over
palladinized charcoal as a catalyst. After removal of the catalyst by filtration,
the filtrate was concentrated by evaporation, whereupon the hydrochloride of
1-p-methoxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylamino-propane
(MP 244°C) crystallized out. For the purpose of demethylation, the 350 grams
of the hydrochloride thus produced were refluxed for 2 hours with 3.5 liters of
aqueous 48% hydrobromic acid. Upon cooling of the reaction solution, 320
grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylaminopropanehydrobromide
(MP 220°C) crystallized out.
·220 grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-
ethylamino-propane hydrobromide were dissolved in 1 liter of methanol, the
resulting solution was boiled with activated charcoal, the charcoal was filtered
off and the filtrate was hydrogenated in the presence of Raney nickel at 60°C
and 5 atmospheres gauge. Thereafter, the catalyst was filtered off, the
methanolic solution was admixed with a small amount of concentrated
hydrobromic acid, and the mixture was evaporated to dryness in vacuo. The
residue was stirred with acetone, the mixture was vacuum filtered and the
filter cake was recrystallized from a mixture of methanol and ether. The 1-phydroxyphenyl-
2-(β-3',5'-dihydroxyphenyl-β-hydroxy)-ethylamino-propane
hydrobromide thus obtained had a melting point of 222° to 223°C.
Therapeutic Function
Bronchodilator
Clinical Use
Fenoterol is an investigational drug in the United States that has been in use in Europe since 1970. It is the p-hydroxyphenyl derivative of metaproteronol, and the combination of the resorcinol ring and the bulky p-hydroxyphenyl isopropyl group on the nitrogen gives fenoterol significant β2-receptor selectivity. It has approximately half the affinity for the β2-receptor as compared to albuterol. The resorcinol ring is resistant to COMT metabolism, and the bulky nitrogen substituent greatly retards MOA metabolism as well giving fenoterol a reasonable oral bioavailability with pharmacokinetics similar to albuterol (i.e., rapid onset and a 4- to 6-hour duration of action after oral inhalation).
Properties of FENOTEROL HYDROBROMIDE
| Melting point: | 226-228°C |
| storage temp. | 2-8°C |
| solubility | Soluble in water and in ethanol (96 per cent). |
| form | neat |
| form | Solid |
| color | White to Off-White |
Safety information for FENOTEROL HYDROBROMIDE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for FENOTEROL HYDROBROMIDE
FENOTEROL HYDROBROMIDE manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Fenoterol hydrobromide CAS 1944-12-3View Details
Fenoterol hydrobromide CAS 1944-12-3View Details
1944-12-3 -
 Fenoterol hydrobromide CAS 1944-12-3View Details
Fenoterol hydrobromide CAS 1944-12-3View Details
1944-12-3 -
 55441-95-7 99%View Details
55441-95-7 99%View Details
55441-95-7 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1 (S)-Methyl 3-amino-2-((tert-butoxycarbonyl)amino)propanote Hydrochloride (DAP-OMe. HCl) 99%View Details
181228-33-1