Fenoterol
- CAS NO.:13392-18-2
- Empirical Formula: C17H21NO4
- Molecular Weight: 303.35
- MDL number: MFCD00242675
- EINECS: 680-817-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-02 08:55:13
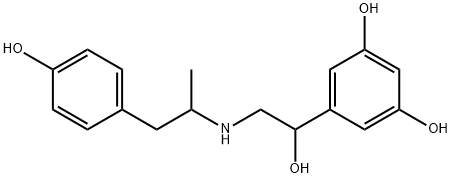
What is Fenoterol?
Toxicity
Symptoms of overdose include angina (chest pain), dizziness, dry mouth, fatigue, flu-like symptoms, headache, heart irregularities, high or low blood pressure, high blood sugar, insomnia, muscle cramps, nausea, nervousness, rapid heartbeat, seizures, and tremor.
Originator
Berotec,Boehringer Ingelheim,W. Germany,1972
The Uses of Fenoterol
antiinflammatory
Background
Fenoterol is an adrenergic beta-2 agonist that is used as a bronchodilator and tocolytic.
Indications
Fenoterol is used for the treatment of asthma.
Definition
ChEBI: A member of the class resorcinols that is 5-(1-hydroxyethyl)benzene-1,3-diol in which one of the methyl hydrogens is replaced by a 1-(4-hydroxyphenyl)propan-2-amino group. A beta2-adrenergic agonist, it is used (as the ydrobromide salt) as a bronchodilator in the management of reversible airway obstruction.
Manufacturing Process
441 grams (1.4 mols) of 3,5-diacetoxy-α-bromo-acetophenone (MP 66°C),
prepared by bromination of 3,5-diacetoxy-acetophenone, were added to a
solution of 714 grams (2.8 mols) of 1-p-methoxyphenyl-2-benzylaminopropane
in 1,000 cc of benzene, and the resulting solution mixture was
refluxed for 1 hour. The molar excess of 1-p-methoxy-phenyl-2-benzylaminopropane
precipitated out as its hydrobromide. After separation of the
precipitated hydrobromide of the amino component, the hydrochloride of 1-pmethoxy-
phenyl-2-(β-3',5'-diacetoxyphenyl-β-oxo)-ethyl-benzylamino-propane
was precipitated from the reaction solution by addition of an ethanolic solution
of hydrochloric acid. The precipitate was separated and, without further
purification, was deacetylated by boiling it in a mixture of 2 liters of aqueous
10% hydrochloric acid and 1.5 liters of methanol.
The resulting solution was filtered through animal charcoal and, after addition
of 2 liters of methanol, it was debenzylated by hydrogenation at 60°C over
palladinized charcoal as a catalyst. After removal of the catalyst by filtration,
the filtrate was concentrated by evaporation, whereupon the hydrochloride of
1-p-methoxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylamino-propane
(MP 244°C) crystallized out. For the purpose of demethylation, the 350 grams
of the hydrochloride thus produced were refluxed for 2 hours with 3.5 liters of
aqueous 48% hydrobromic acid. Upon cooling of the reaction solution, 320
grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylaminopropanehydrobromide
(MP 220°C) crystallized out.
·220 grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-
ethylamino-propane hydrobromide were dissolved in 1 liter of methanol, the
resulting solution was boiled with activated charcoal, the charcoal was filtered
off and the filtrate was hydrogenated in the presence of Raney nickel at 60°C
and 5 atmospheres gauge. Thereafter, the catalyst was filtered off, the
methanolic solution was admixed with a small amount of concentrated
hydrobromic acid, and the mixture was evaporated to dryness in vacuo. The
residue was stirred with acetone, the mixture was vacuum filtered and the
filter cake was recrystallized from a mixture of methanol and ether. The 1-phydroxyphenyl-
2-(β-3',5'-dihydroxyphenyl-β-hydroxy)-ethylamino-propane
hydrobromide thus obtained had a melting point of 222° to 223°C.
brand name
Berotec [as hydrobromide](Boehringer Ingelheim);Dosberotec;Duovent;Fensol;Partusisten.
Therapeutic Function
Bronchodilator
World Health Organization (WHO)
Fenoterol, a beta 2-adrenoreceptor agonist with bronchodilator activity, was introduced in 1971 for the management of asthma. In the 1960's, the use of other sympathomimetics in pressurised aerosols had already been associated with an increase in mortality due to asthma. However, it was not clear whether patients died from the severity of the asthma attack or from its treatment.
Mechanism of action
Fenoterol is a selective stimulant of β2-adrenoreceptors. It dilates bronchi and blood vessels, has a pronounced tocolytic action, lowers contractile activity and reduces uterus tonicity. It is mainly used in premature births.
Pharmacokinetics
Fenoterol is a beta agonist designed to open up the airways to the lungs by decreasing bronchconstriction.
Clinical Use
Fenoterol is a selective β2-sympathomimetic agent that is in wide clinical use in Europe.
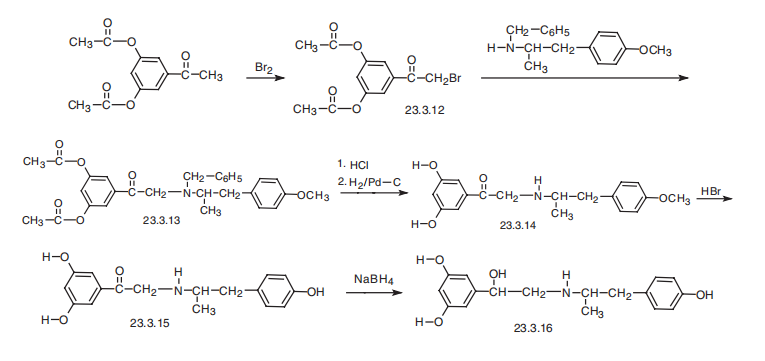
Synthesis
Fenoterol, 3,5-dihydroxy-|á[[(-p-hydroxy-|á-methylphenethyl)amino]methyl]- benzyl alcohol (23.3.16), is synthesized from 3,5-diacetoxyacetophenone, which is brominated to give 3,5-diacetoxybromacetophenone (23.3.12). This is reacted with 2-benzylamino-1-(4-methoxyphenyl)-propane, giving the corresponding tertiary amine 23.3.13. Hydrolysis of the acetyl group of this product and removal of the protective benzyl group by hydrogen reduction using a palladium on carbon catalyst gives a secondary amine 23.3.14. This is reacted with hydrobromic acid, which cleaves the ether bond in the benzene ring, producing phenol derivative 23.3.15. Finally, reduction of the carbonyl group with hydrogen gives the desired fenoterol (23.3.16).
Metabolism
Hepatic.
Properties of Fenoterol
| Melting point: | 181-183°C |
| Boiling point: | 566.0±45.0 °C(Predicted) |
| Density | 1.289±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| pka | pKa 8.5 (Uncertain);10.0 (Uncertain) |
| form | Solid |
| color | Light brown to brown |
| CAS DataBase Reference | 13392-18-2(CAS DataBase Reference) |
Safety information for Fenoterol
Computed Descriptors for Fenoterol
Abamectin manufacturer
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran





You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4