Fenofibric acid
Synonym(s):2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid;Fenofibric acid
- CAS NO.:42017-89-0
- Empirical Formula: C17H15ClO4
- Molecular Weight: 318.75
- MDL number: MFCD00792461
- EINECS: 255-626-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
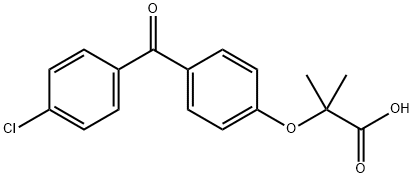
What is Fenofibric acid?
Absorption
Some studies have demonstrated that the bioavailability of fenofibric acid (a sample administration of 130 mg oral suspension to healthy volunteers about 4 hours after a light breakfast) is approximately 81% in the stomach, 88% in the proximal small bowel, 84% in the distal small bowel, and 78% in the colon . Nevertheless, following the oral administration of fenofibric acid in healthy volunteers, median peak plasma levels for the drug occurred about 2.5 hours after administration . Moreover, exposure after administration of three 35 mg fenofibric acid tablets is largely comparable to that of one 105 mg tablet .
Chemical properties
White to Off-White Solid
The Uses of Fenofibric acid
Fenofibric acid, the active metabolite of fenofibrate. Increases Apolipoprotein A-Iediated High-Density Lipoprotein Biogenesis by enhancing transcription of ATP-Binding cassette transporter A1 gene in a liver X receptorependent manner
The Uses of Fenofibric acid
Fenofibric acid, the active metabolite of fenofibrate. Increases Apolipoprotein A-I–Mediated High-Density Lipoprotein Biogenesis by enhancing transcription of ATP-Binding cassette transporter A1 gene in a liver X receptor–dependent manner.
The Uses of Fenofibric acid
DPP4 inhibitor, antidiabetic
Background
Fenofibric acid is a lipid-lowering agent that is used in severe hypertriglyceridemia, primary hyperlipidemia, and mixed dyslipidemia. It works to decrease elevated low-density lipoprotein cholesterol, total cholesterol, triglycerides, apolipoprotein B, while increasing high-density lipoprotein cholesterol. Due to its high hydrophilicity and poor absorption profile, prodrug ,fenofibrate, and other conjugated compounds of fenofibric acid, such as choline fenofibrate, have been developed for improved solubility, gastrointestinal absorption, and bioavailability, and more convenient administration.
Indications
For use as an adjunctive therapy to diet to: (a) reduce triglyceride levels in adult patients with severe hypertriglyceridemia, and (b) reduce elevated total cholesterol, low-density-lipoprotein (LDL-C), triglycerides, and apolipoprotein B, and to increase high-density-lipoprotein (HDL-C) in adult patients with primary hypercholesterolemia or mixed dyslipidemia (Fredrickson Types IIa and IIb).
What are the applications of Application
Fenofibric Acid is the active metabolite of fenofibrate
Definition
ChEBI: A monocarboxylic acid that is 2-methylpropanoic acid substituted by a 4-(4-chlorobenzoyl)phenoxy group at position 2. It is a metabolite of the drug fenofibrate.
Pharmacokinetics
Various clinical studies have shown that elevated levels of total cholesterol, low-desnsity-lipoprotein (LDL-C), and apolipoprotein B (apo B) - an LDL membrane complex - are associated with human atherosclerosis . Concurrently, decreased levels of high-density-lioprotein (HDL-C) and its transport complex, apolipoproteins apo AI and apo AII, are associated with the development of atherosclerosis . Furthermore, epidemiological investigations demonstrate that cardiovascular morbidity and mortality vary directly with the levels of total cholesterol, LDL-C, and triglycerides, and inversely with the level of HDL-C .
Fenofibric acid, the active metabolite of fenofibrate, subsequently produces reductions in total cholesterol, LDL-C, apo B, total triglycerides, and triglyceride rich lipoprotein (VLDL) in treated patients . Moreover, such treatment with fenofibrate also results in increases in HDL-C and apo AI and apo AII .
Metabolism
In vitro and in vivo metabolism studies reveal that fenofibric acid does not experience significant oxidative metabolism via the cytochrome P450 isoenzymes . The CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 enzymes are not known to play a role in the metabolism of fenofibric acid .
Rather, fenofibric acid is predominantly conjugated with glucuronic acid and then excreted in urine . A small amount of fenofibric acid is reduced at the carbonyl moiety to benzhydrol metabolite which is, in turn, conjugated with glucuronic acid and excreted in urine .
Properties of Fenofibric acid
| Melting point: | 177-179°C |
| Boiling point: | 486.5±35.0 °C(Predicted) |
| Density | 1.286±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | powder |
| pka | 3.09±0.10(Predicted) |
| color | White to Off-White |
| BRN | 2058973 |
| CAS DataBase Reference | 42017-89-0(CAS DataBase Reference) |
Safety information for Fenofibric acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Fenofibric acid
Fenofibric acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


![FENOFIBRATE RELATED COMPOUND C (25 MG) (1-METHYLETHYL 2-[[2-[4-(4-CHLOROBENZOYL)PHENOXY]-2-METHYLPROPANOYL]OXY]-2-METHYLPROPANOATE)](https://img.chemicalbook.in/CAS/GIF/217636-48-1.gif)
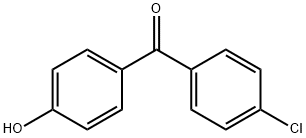
![(4-Chlorophenyl)[4-(1-Methylethoxy)phenyl]Methanone
(Fenofibrate IMpurity)](https://img.chemicalbook.in/CAS/GIF/154356-96-4.gif)
![2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid methyl ester](https://img.chemicalbook.in/CAS/GIF/42019-07-8.gif)
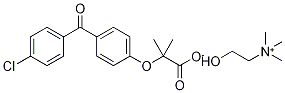
![3-[4-(4-Chlorobenzoyl)phenoxy]-2-butanone
(Fenofibrate IMpurity)](https://img.chemicalbook.in/CAS/GIF/217636-47-0.gif)
You may like
-
 42017-89-0 Fenofibrate EP Impurity B 98%View Details
42017-89-0 Fenofibrate EP Impurity B 98%View Details
42017-89-0 -
 42017-89-0 98%View Details
42017-89-0 98%View Details
42017-89-0 -
 Fenofibric acid 98%View Details
Fenofibric acid 98%View Details -
 Fenofibric Acid CAS 42017-89-0View Details
Fenofibric Acid CAS 42017-89-0View Details
42017-89-0 -
 Fenofibric acid 98% CAS 42017-89-0View Details
Fenofibric acid 98% CAS 42017-89-0View Details
42017-89-0 -
![2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid CAS 42017-89-0](https://img.chemicalbook.in//Content/image/CP5.jpg) 2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid CAS 42017-89-0View Details
2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid CAS 42017-89-0View Details
42017-89-0 -
 Fenofibric Acid PowderView Details
Fenofibric Acid PowderView Details
42017-89-0 -
 99% Fenofibric Acid (working standard), Analytical GradeView Details
99% Fenofibric Acid (working standard), Analytical GradeView Details
42017-89-0
