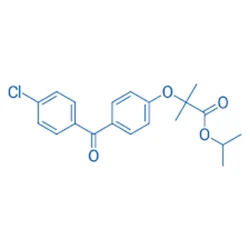
Fenofibrate
Synonym(s):2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropanoic acid isopropyl ester;FEN
- CAS NO.:49562-28-9
- Empirical Formula: C20H21ClO4
- Molecular Weight: 360.83
- MDL number: MFCD00133314
- EINECS: 256-376-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04

What is Fenofibrate?
Absorption
A single 300mg oral dose of fenofibrate reaches a Cmax of 6-9.5mg/L with a Tmax of 4-6h in healthy, fasting volunteers.
Toxicity
The oral LD50 in rats is >2g/kg and in mice is 1600mg/kg. The oral TDLO in rats is 9mg/kg.
Treat patients with supportive care including monitoring of vital signs and observing clinical status. Recent overdose may be treated with inducing vomiting or gastric lavage. Due to fenofibrate's extensive protein binding, hemodialysis is not expected to be useful.
Chemical properties
Crystalline Solid
Originator
Lipantyl,Fournier,France,1975
The Uses of Fenofibrate
Fenofibrate has been used:
- to study its effect on plasma lipids, liver phenotype and gene expression
- to study its impact on endothelium-dependent vasodilatation of thoracic aorta
- to administer to NASH knock-in mice along with a high fat diet (HFD) to study its effect
The Uses of Fenofibrate
Antilipemic. It is a lipid regulating drug. Increases high density lipoprotein levels by reducing cholesteryl ester transfer protein expresion.
The Uses of Fenofibrate
antihyperlipidemic
The Uses of Fenofibrate
Cardiovascular disease
Indications
Fenofibrate is indicated as adjunctive therapy to diet to reduce elevated LDL-C, Total-C, Triglycerides, and Apo B, and to increase HDL-C adults with primary hypercholesterolemia or mixed dyslipidemia. Fenofibrate is also indicated to treat adults with severe hypertriglyceridemia.
What are the applications of Application
Fenofibrate is a hypolipidemic PPARα agonist
Background
Fenofibrate is a fibric acid derivative like clofibrate and gemfibrozil. Fenofibrate is used to treat primary hypercholesterolemia, mixed dyslipidemia, severe hypertriglyceridemia.
Fenofibrate was granted FDA approval on 31 December 1993.
Definition
ChEBI: A chlorobenzophenone that is (4-chlorophenyl)(phenyl)methanone substituted by a [2-methyl-1-oxo-1-(propan-2-yloxy)propan-2-yl]oxy group at position 1 on the phenyl ring.
Manufacturing Process
(a) Preparation of p-(4-chlorobenzoyl)-phenoxyisobutyric acid: 1 mol of 4-
hydroxy-4'-chlorobenzophenone is dissolved in anhydrous acetone and then 5
mols of powdered sodium hydroxide is added. The corresponding sodium
phenoxide precipitates. Refluxing is effected, and then, 1.5 mols of CHCl3
diluted with anhydrous acetone is added and the resulting mixture is refluxed
for 10 hours. After cooling, water is added, the acetone is evaporated, the
aqueous phase is washed with ether and acidified and the organic phase is
redissolved in ether and extracted into a solution of bicarbonate. The
bicarbonate solution is than acidified to obtain the desired acid, having a
melting point of 185°C, with a yield of 75%.
(b) Preparation of fenofibrate: 1 mol of the acid obtained is converted into its
acid chloride using thionyl chloride (2.5 mols). 1 mol of the acid chloride is
then condensed with 1.05 mol of isopropyl alcohol in the presence of 0.98 mol
of pyridine in an inert solvent such as benzene.
Since traces of SO2 (which has a bad smell) may be obtained from the thionyl
chloride, it is preferable to avoid this disadvantage by carrying out the
esterification directly.
Therapeutic Function
Antihyperlipoproteinemic
General Description
Fenofibrate, 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoic acid 1-methylethyl ester (Tricor),has structural features represented in clofibrate. The primarydifference involves the second aromatic ring. This imparts agreater lipophilic character than exists in clofibrate, resultingin a much more potent hypocholesterolemic and triglycerideloweringagent. Also, this structural modification results in alower dose requirement than with clofibrate or gemfibrozil.
Biochem/physiol Actions
Fenofibrate increases high density lipoprotein levels by reducing cholesteryl ester transfer protein expression. It is used in treating the condition of increased cholesterol levels in the blood, abnormal lipid levels in the body and also hypertriglyceridaemia. Fenofibrate is a lipid regulating drug and proliferator-activated receptor-α (PPARα) mediates its action. It decreases the plasma levels of fibrinogen and C-reactive protein. By this fenofibrate allows better flow-mediated dilatation and reduces the risk of atherosclerosis. Fenofibrate is also known to reduce uric acid levels.
Pharmacokinetics
Fenofibrate is a fibrate that activates peroxisome proliferator activated receptor alpha (PPARα) to alter lipid metabolism and treat primary hypercholesterolemia, mixed dyslipidemia, and severe hypertriglyceridemia. Fenofibrate requires once daily dosing and has a half life of 19-27 hours so its duration of action is long. Fenofibrate capsules are given at a dose of 50-150mg daily so the therapeutic index is wide. Patients should be counselled about the risk of rhabdomyolysis, myopathy, and cholelithiasis when taking fibrates.
Drug interactions
Potentially hazardous interactions with other drugs Antibacterials: increased risk of myopathy with daptomycin - try to avoid concomitant use. Anticoagulants: enhances effect of coumarins and phenindione; dose of anticoagulant should be reduced by up to 50% and readjusted by monitoring INR. Antidiabetics: may improve glucose tolerance and have an additive effect with insulin or sulphonylureas. Ciclosporin: ciclosporin levels appear to be unaffected; however, it is recommended that concomitant therapy should be avoided because of the possibility of elevated serum creatinine levels. Colchicine: possible increased risk of myopathy. Lipid-regulating drugs: increased risk of myopathy in combination with statins and ezetimibe (maximum 20 mg of rosuvastatin); increased risk of cholelithiasis and gallbladder disease with ezetimibe - avoid with ezetimibe.
Metabolism
Fenofibrate is completely hydrolyzed by liver carboxylesterase 1 to fenofibric acid. Fenofibric acid is either glucuronidated or has its carbonyl group reduced to a benzhydrol that is then glucuronidated. Glucuronidation of fenofibrate metabolites is mediated by UGT1A9. Reduction of the carbonyl group is primarily mediated by CBR1 and minorly by AKR1C1, AKR1C2, AKR1C3, and AKR1B1.
Metabolism
After oral administration, fenofibrate is rapidly hydrolysed by esterases to the active metabolite fenofibric acid.No unchanged fenofibrate can be detected in the plasma. Fenofibric acid is excreted mainly in the urine, mainly as the glucuronide conjugate, but also as a reduced form of fenofibric acid and its glucuronide; practically all the drug is eliminated from the body within 6 days
Storage
Store at RT
Properties of Fenofibrate
| Melting point: | 80-810C |
| Boiling point: | 469.8±35.0 °C(Predicted) |
| Density | 1.177±0.06 g/cm3(Predicted) |
| storage temp. | room temp |
| solubility | Practically insoluble in water, very soluble in methylene chloride, slightly soluble in ethanol (96 per cent) |
| form | powder |
| color | off-white |
| Water Solubility | 0.8mg/L(25 ºC) |
| Merck | 14,3978 |
| InChI | InChI=1S/C20H21ClO4/c1-13(2)24-19(23)20(3,4)25-17-11-7-15(8-12-17)18(22)14-5-9-16(21)10-6-14/h5-13H,1-4H3 |
| EPA Substance Registry System | Propanoic acid, 2-[4-(4-chlorobenzoyl)phenoxy]-2-methyl-, 1-methylethyl ester (49562-28-9) |
Safety information for Fenofibrate
| GHS Hazard Statements |
H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P501:Dispose of contents/container to..… |
Computed Descriptors for Fenofibrate
| InChIKey | YMTINGFKWWXKFG-UHFFFAOYSA-N |
| SMILES | C(OC(C)C)(=O)C(OC1=CC=C(C(=O)C2=CC=C(Cl)C=C2)C=C1)(C)C |
Fenofibrate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
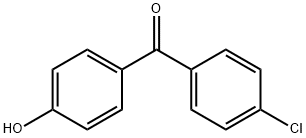

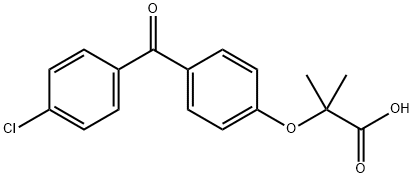
![FENOFIBRATE RELATED COMPOUND C (25 MG) (1-METHYLETHYL 2-[[2-[4-(4-CHLOROBENZOYL)PHENOXY]-2-METHYLPROPANOYL]OXY]-2-METHYLPROPANOATE)](https://img.chemicalbook.in/CAS/GIF/217636-48-1.gif)
![(4-Chlorophenyl)[4-(1-Methylethoxy)phenyl]Methanone
(Fenofibrate IMpurity)](https://img.chemicalbook.in/CAS/GIF/154356-96-4.gif)
![2-[4-(4-Chlorobenzoyl)phenoxy]-2-methylpropionic acid methyl ester](https://img.chemicalbook.in/CAS/GIF/42019-07-8.gif)
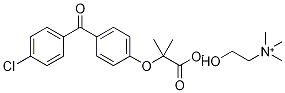
![3-[4-(4-Chlorobenzoyl)phenoxy]-2-butanone
(Fenofibrate IMpurity)](https://img.chemicalbook.in/CAS/GIF/217636-47-0.gif)
You may like
-
 Fenofibrate 99%View Details
Fenofibrate 99%View Details -
 Fenofibrate 98%View Details
Fenofibrate 98%View Details -
 Fenofibrate 98% CAS 49562-28-9View Details
Fenofibrate 98% CAS 49562-28-9View Details
49562-28-9 -
 White Fenofibrate PowderView Details
White Fenofibrate PowderView Details
49562-28-9 -
 Fenofibrate Powder API MANUFACTURER INDIAView Details
Fenofibrate Powder API MANUFACTURER INDIAView Details
49562-28-9 -
 Finofibrate Chemical IHRSView Details
Finofibrate Chemical IHRSView Details
49562-28-9 -
 Finofibrate Api powderView Details
Finofibrate Api powderView Details
49562-28-9 -
 FenofibrateView Details
FenofibrateView Details
49562-28-9
