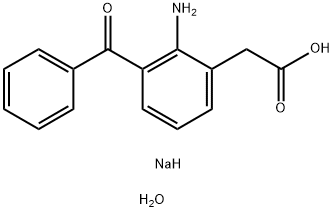
Fenazox
Synonym(s):Azoxybenzene;Fenazox
- CAS NO.:61618-27-7
- Empirical Formula: C15H16NNaO4
- Molecular Weight: 297.29
- MDL number: MFCD09842317
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is Fenazox?
Description
Amfenac sodium is a non-steroidal antiinflammatory agent structurally related to ketoprofen,suprofen(10) and tiaprofenic acid.It is reported to be effective in the short term treatment of rheumatoidarthritis,osteoarthritis and pain associated with minor surgical procedures.
Chemical properties
Yellow Solid
Originator
A.H. Robins (USA)
The Uses of Fenazox
Fenazox is used in the synthetic preparation of amides via reductive amidation of esters, which is used in the synthesis of bio-active molecules and natural products.
The Uses of Fenazox
Amfenac is an antibacterial agent
What are the applications of Application
Amfenac Sodium Salt is an antibacterial agent
Definition
ChEBI: A hydrate that is the monohydrate of the sodium salt of amfenac.
Manufacturing Process
7-Benzoylindolin-2-one:
Method A. A mixture of 2.5 g (0.0077 mole) of ethyl 2-acetamido-3-
benzoylphenylacetate in 50 ml of 3 N hydrochloric acid was refluxed for one
hour. The reaction mixture was filtered and the filtrate was poured into a
mixture of ice and water. The precipitate was collected and recrystallized from
acetone; yield of 7-benzoylindolin-2-one 1 g (55%); melting point 154°C.
Method B. A solution of 1.3 g (0.0044 mole) of 2-acetamido-3-
benzoylphenylacetic acid in 15 ml of 3 N hydrochloric acid and 15 ml of acetic
acid was refluxed for three hours. The cooled solution was poured into ice
water and the 7-benzoylindolin-2-one which precipitated was collected and
dried.
2-Amino-3-benzoylphenylacetic acid:
A mixture of 1.0 g (0.004 mole) of 7-benzoylindolin-2-one was added to 30
ml of 3 N sodium hydroxide and the basic solution was refluxed for 45 min
under nitrogen. The mixture was filtered and the filtrate was neutralized with
glacial acetic acid. The precipitate was filtered off, washed with water and
dried. The 2-amino-3-benzoylbenzeneacetic acid melted at 122°C (dec.). The
yield was 0.8 g (72%).
brand name
FENAZOX
Therapeutic Function
Antiinflammatory
General Description
Amfenac (Fenazox), its amide prodrug, nepafenac (Nevanac),and the related analog, bromofenac, are amphoteric becauseof the presence of an additional aromatic amine group.They are less likely to be absorbed into the general circulation.They are approved for use as topical ocular anti-inflammatoryagents for the treatment of postoperative ocular pain,inflammation, and posterior segment edema. The only observedside effects of these drugs are all related to tissuesaround the eye including abnormal ocular sensation, eye rednessand irritation, burning and stinging, and conjunctival orcornea edema.
Synthesis
The reaction of 1-aminoindolin- 2-one with phenylacetone in presence of acetic acid in refluxing ethanol gives 1-(α- methylphenethylidieneimino)indolin-2-one, which by reaction with refluxing ethanolic hydrogen chloride affords ethyl α-(2-methyl- 3-phenylindol-7-yl)acetate. The ozonolysis of this intermediate in acetic acid yields ethyl 2- acetamido-3-benzoylphenyl acetate, which is cyclized by refluxing with HCl in acetic acid to give 7-benzoylindolin-2-one. Alternatively, the hydrolysis of the ester ethyl α-(2-methyl-3- phenylindol-7-yl)acetate with KOH in refluxing water affords the corresponding acid, which can be ozonolyzed as before yielding 2-acetamido- 3-benzoylphenylacetic acid. This acid can be cyclized to 7-benzoyl-1,3-dihydro-indol-2-one by refluxing with HCl in acetic acid as before .
Properties of Fenazox
| Melting point: | 242-244°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | Yellow |
Safety information for Fenazox
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Fenazox
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

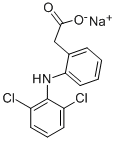

You may like
-
 61618-27-7 Amfenac sodium 99%View Details
61618-27-7 Amfenac sodium 99%View Details
61618-27-7 -
 Sodium 2-(2-amino-3-benzoylphenyl)acetate hydrate 95% CAS 61618-27-7View Details
Sodium 2-(2-amino-3-benzoylphenyl)acetate hydrate 95% CAS 61618-27-7View Details
61618-27-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8