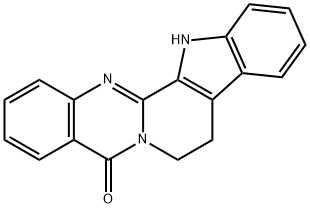
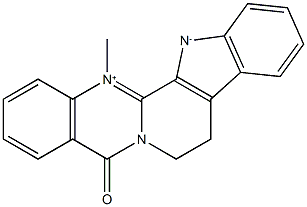
Evodiamine
- CAS NO.:518-17-2
- Empirical Formula: C19H17N3O
- Molecular Weight: 303.36
- MDL number: MFCD06407824
- EINECS: 637-111-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
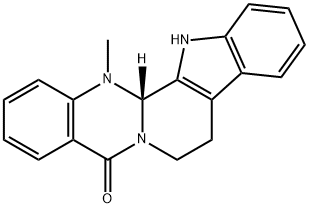
What is Evodiamine?
Description
Evodiamine (518-17-2) is a quinolone alkaloid isolated from Evodia rutaecarpa.1 Evodia Rutaecarpa is a very popular herb used in traditional chinese medicine to treat a variety of ailments. Evodiamine has been shown to elicit a variety of biological effects including induction of apoptosis, inhibiting cancer cell proliferation, promoting cell cycle arrest in G2/M, inhibiting invasion and metastasis of cancer cells, inhibiting angiogenesis, induction of oxidative stress and subsequent apoptosis and inhibition of NF-κB.2 It also functions as an activator of TRPV1 (EC50 = 1.03 μM)3 and acts as an anti-inflammatory agent4.
Chemical properties
White powder
The Uses of Evodiamine
A chemical extracted from Evodia plants, and has been shown to reduce fat uptake.
The Uses of Evodiamine
An antiangiogenic and antiinflammatory agonist of TRPV1.
The Uses of Evodiamine
A vanilloid receptor agonist that induces apoptosis in leukemia U937 cells
What are the applications of Application
Evodiamine is an antiangiogenic and antiinflammatory agonist of TRPV1
Definition
ChEBI: Evodiamine is a member of beta-carbolines.
Anticancer Research
The fruit of Evodia rutaecarpa Benth. is a standout among the most prominent andmultipurpose herbs utilized in China for pharmacological application (Fei et al.2003). Phytochemical analysis demonstrated that evodiamine, an indole alkaloid,exists in elevated amounts in Chinese herb evodia. This has wide-ranging biologicalactivities with vasodilatory, anti-obesity, anticancer, and anti-inflammatoryeffects (Jiang and Hu 2009). Evodiamine represses PDGF-BB-induced vascularsmooth muscle cell proliferation in a dose-dependent manner. It inhibits cell cycleprogression by suppressing the activation of p38 and Erk/MAPK pathway andameliorates ROS generation (Yang et al. 2008b; Heo et al. 2009; Yang et al. 2013).Restraint of mitogenesis and p38/p Erk action may fill in as the molecular basis forthe capacity of evodiamine. Evodiamine causes continued initiation of Erk/MAPKsignaling pathway in 3T3-L1 and essential preadipocytes prompting a potentinhibitory impact for adipogenesis (Wang et al. 2008). The consequence of thisinvestigation showed that evodiamine hindered vascular smooth muscle cell(VSMC) multiplication by stifling cell cycle movement, p38 MAPK and Erk 1/2enactment, and generation of ROS. Moreover, evodiamine offers the potential oftreating cardiovascular infections connected with the strange expansion of vascularsmooth muscle cells.
References
1) Shoji et al. (1986) Isolation of evodiamine, a powerful cardiotonic principle, from Evodia Rutaecarpa Bentham (Rutaceae); J. Pharm. Sci. 75 612 2) Jiang and Hu et al. (2009) Evodiamine: A novel anti-cancer alkaloid from Evodia rutaecarpa; Molecules, 14 1852 3) Pearce et al. (2004) Evodiamine functions as an agonist for the vanilloid receptor; Org. Biomol. Chem., 2 2281 4) Choi et al. (2006) Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms; Arch. Pharm. Res., 29 293
Properties of Evodiamine
| Melting point: | 278° |
| Boiling point: | 444.29°C (rough estimate) |
| alpha | D15 +352° (acetone); D +440° (chloroform) |
| Density | 1.1684 (rough estimate) |
| refractive index | 1.5900 (estimate) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | Soluble in DMSO (up to 5 mg/ml). |
| pka | 17.27±0.20(Predicted) |
| form | Off-white to light yellow solid. |
| color | Yellow |
| Odor | Odorless |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 2 months. |
| CAS DataBase Reference | 518-17-2(CAS DataBase Reference) |
Safety information for Evodiamine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Evodiamine
| InChIKey | TXDUTHBFYKGSAH-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Evodiamine, 98% CAS 518-17-2View Details
Evodiamine, 98% CAS 518-17-2View Details
518-17-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8