Etonogestrel
Synonym(s):(17α)-13-Ethyl-17-hydroxy-11-methylene-18,19-dinorpregn-4-en-20-yn-3-one;3-Keto-desogestrel;3-Oxo-desogestrel;Etonogestrel
- CAS NO.:54048-10-1
- Empirical Formula: C22H28O2
- Molecular Weight: 324.46
- MDL number: MFCD00867863
- EINECS: 258-936-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
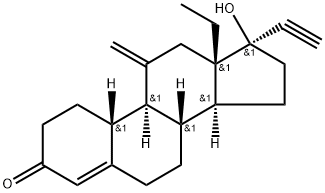
What is Etonogestrel?
Absorption
Vaginal administration of etonogestrel is known to be significantly absorbed through the vaginal epithelium but it does not increase the levels of etonogestrel in the urine. On the other hand, oral administration is absorbed in the GI tract and it goes through the first-pass metabolism.
When etonogestrel is administered subdermally it is absorbed rapidly into the bloodstream and it presents a bioavailability of 82%. It is reported that the implant releases around 60 mcg per day in the first 3 months and then decreases steady reaching a concentration of 30 mcg at the end of year 2.
Toxicity
The reported LD50 of oral etonogestrel in the rat is reported to be higher than 2000 mg/kg. Overdosage can only happen when more than one implant is inserted. In cases of overdose, removal of the implant is recommended.
There aren't reports relating etonogestrel with carcinogenesis, mutagenesis or impaired fertility.
Description
Etonogestrel molecule is a 3-ketodesogestrel or 19-nortestosterone which is a synthetic biologically active metabolite of progestin desogestrel. The first product including etonogestrel was developed by the Merck subsidiary Organon and FDA approved in 2001.
Chemical properties
White Solid
The Uses of Etonogestrel
A biologically active metabolite of Desogestrel.
Background
Etonogestrel molecule is a 3-ketodesogestrel or 19-nortestosterone which is a synthetic biologically active metabolite of progestin desogestrel. The first product including etonogestrel was developed by the Merck subsidiary Organon and FDA approved in 2001.
Indications
Etonogestrel is administered in subdermal implants as long-acting reversible contraception. It is known to be effective in postpartum insertion including breastfeeding women.
Etonogestrel is part of the long-acting contraceptive implants that prevent pregnancy. The implant's effect can remain for 5 years.
What are the applications of Application
Etonogestrel is an active metabolite of Desogestrel
Definition
ChEBI: Etonogestrel is a 17beta-hydroxy steroid, a 3-oxo-Delta(4) steroid and a terminal acetylenic compound. It has a role as a contraceptive drug, a progestin and a female contraceptive drug.
brand name
Implanon (Organon).
General Description
Etonogestrel, 17α-13-ethyl-17hydroxy-11-methylene-18,19-dinorpregn-4-en-20-yn-3-one,3-ketodesogestrel, is the active metabolite of desogestrel. It isthe progestin component in a newer implantable contraceptive(Implanon) and in the vaginal contraceptive ring (NuvaRing).
Biochem/physiol Actions
Etonogestrel is a lipophilic molecule. Pharmacokinetics studies reveal that etonogestrel is bound to protein albumin in the blood. This remains independent of both endogenous and exogenous concentration of estradiol.
Pharmacokinetics
Etonogestrel attains its therapeutic effect inhibiting fertility by impairing the release of the luteinizing hormone which is one of the most important reproductive hormones for ovulation. As well, etonogestrel is known to increase the viscosity of the cervical mucus hindering the passage of the spermatozoa and altering the lining in the uterus to prevent the implantation of the fertilized eggs in the endometrium.
In clinical trials, etonogestrel was implanted and reported to avoid 100% of pregnancies over a three year period. When the implant was removed, normal periods were reinstalled within 90 days in 91% of the individuals. Fertility was established quickly with 20 reported pregnancies within 3 months of implant removal.
The implants of etonogestrel release 40 mcg of etonogestrel daily and they usually provide a continuous contraception effect for 3 years. When the implant is administered, the failure rate is reported to be 0.1%. Some non-contraceptive effects are improved dysmenorrhea. All data of etonogestrel comes from patients between 80-130% of the body mass.
Metabolism
Etonogestrel is highly metabolized in the liver by the action of the cytochrome isoenzyme 3A4 mainly by the presence of hydroxylation, sulfate conjugation and glucuronide conjugation reactions.
Mode of action
Etonogestrel binds to the cytoplasmic progesterone receptors in the reproductive system and subsequently activates progesterone receptor mediated gene expression. As a result of the negative feedback mechanism, luteinizing hormone (LH) release is inhibited, which leads to an inhibition of ovulation and an alteration in the cervical mucus and endometrium.
Properties of Etonogestrel
| Melting point: | 182-184°C |
| Boiling point: | 473.1±45.0 °C(Predicted) |
| alpha | D +87.6° |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: >5mg/mL |
| form | powder |
| pka | 13.04±0.40(Predicted) |
| color | white to beige |
| optical activity | [α]/D +80 to +95°, c = 1 (CHCl3) |
| CAS DataBase Reference | 54048-10-1(CAS DataBase Reference) |
Safety information for Etonogestrel
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H351:Carcinogenicity H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Etonogestrel
| InChIKey | GCKFUYQCUCGESZ-BPIQYHPVSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Etonogestrel CAS 54048-10-1View Details
Etonogestrel CAS 54048-10-1View Details
54048-10-1 -
 Desogestrel Related Compound C CAS 54048-10-1View Details
Desogestrel Related Compound C CAS 54048-10-1View Details
54048-10-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8