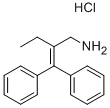
Etifelmine
- CAS NO.:341-00-4
- Empirical Formula: C17H19N
- Molecular Weight: 237.34
- MDL number: MFCD00868094
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
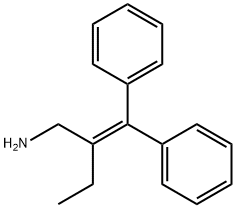
What is Etifelmine?
Originator
Etifelmine,Giulini,W. Germany,1963
Definition
ChEBI: Etifelmine is a diarylmethane.
Manufacturing Process
(a) Preparation of 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine: 10 g of 2-
ethyl-3-hydroxy-3,3-diphenyl-propionitrile are dissolved in 200 ml of
methanol. 10 ml of acetic acid are added to the mixture, and the mixture is
hydrogenated in the presence of platinum as catalyst. After the hydrogen
uptake or consumption has ceased, the reaction is interrupted, the catalyst is
filtered off and the filtrate is evaporated in vacuo to dryness. The residue is
dissolved in water and, after the addition of 1 ml of hydrochloric acid, the
solution extracted with ether. The acidified ether-phase is discarded. The
aqueous phase is made alkaline with ammonia, whereby the base crystallizesout. The crystals are recovered and recrystallized from methanol. The melting
point of the 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine thereby obtained is
132°C.
(b) Preparation of 2-ethyl-3,3-diphenyl-1-amino-propene-(2)-hydrochloride: 5
g of 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine are dissolved in 50 ml of
acetic acid. Gaseous hydrogen chloride is passed through the solution for 10
minutes, and thereafter the solution is boiled for one hour under reflux. The
solution is then distilled to dryness. The residue is dissolved in water and the
acidified solution extracted with ether. The aqueous phase is separated, made
alkaline with ammonia and extracted with ether. The ether phase is dried over
sodium sulfate, the ether distilled off and the residue is dissolved in
methanolic hydrogen chloride. On the addition of absolute ether, the
hydrochloride of 2-ethyl-3,3-diphenyl-1-amino-propene-(2) is crystallized out.
The crystalline substance thereby obtained has a melting point of 232°C.
Therapeutic Function
Central stimulant, Antihypotensive
Safety information for Etifelmine
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




![6-BROMO-3-[(1E)-ETHANEHYDRAZONOYL]-4-PHENYLQUINOLIN-2(1H)-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure3/GIF/CB2420213.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1