Florfenicol
Synonym(s):[R-(R*,S*)]-2,2-Dichloro-N-[1-(fluoromethyl)-2-hydroxy-2-[4-(methylsulfonyl)phenyl]ethyl]acetamide;Aquafen;Nuflor;SCH-25298
- CAS NO.:73231-34-2
- Empirical Formula: C12H14Cl2FNO4S
- Molecular Weight: 358.21
- MDL number: MFCD00864834
- EINECS: 642-986-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-25 18:47:31
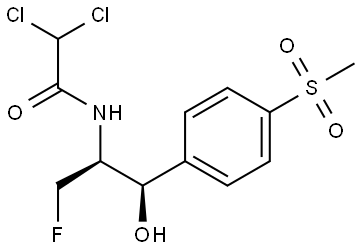
What is Florfenicol?
Description
Florfenicol is a broad-spectrum fluorinated antibiotic and a derivative of thiamphenicol . It is active against human clinical isolates of enteric bacteria, including E. coli, Klebsiella, Enterobacter, Citrobacter, P. mirabilis, and Salmonella (MIC50s = 6.3-12.5 μg/ml). Florfenicol is also active against clinical isolates of various bovine and porcine respiratory tract pathogens, including P. multocida, A. pleuropneumoniae, and B. bronchiseptica (MIC50s = 0.25-4 μg/ml). It inhibits peptidyl transferase activity in 70S ribosomes isolated from E. coli when used at a concentration of 1 mM. Formulations containing florfenicol have been used in the treatment of infectious respiratory disease in cattle.
Chemical properties
White or off-white crystalline powder, odorless. Easily soluble in dimethylformamide, soluble in methanol, slightly soluble in glacial acetic acid, very slightly soluble in water or chloroform.
The Uses of Florfenicol
Florfenicol is a fluorinated derivative of thiamphenicol. It is a protein synthesis inhibitor that inhibits bacterial protein synthesis by binding to ribosome 50S and 70S subunits. Florfenicol is used as an antibacterial.
What are the applications of Application
Florfenicol is a broad-spectrum antibacterial preparation
Definition
ChEBI: Florfenicol is a carboxamide that is the N-dichloroacetyl derivative of (1R,2S)-2-amino-3-fluoro-1-[4-(methanesulfonyl)phenyl]propan-1-ol. A synthetic veterinary antibiotic that is used for treatment of bovine respiratory disease and foot rot; also used in aquaculture. It has a role as an antimicrobial agent. It is a sulfone, a secondary alcohol, an organofluorine compound, an organochlorine compound and a secondary carboxamide. It is functionally related to a dichloroacetic acid.
Preparation
Florfenicol is synthesised from thiamphenicol by replacing the 3-hydroxy group with fluorine, first synthesised at Schering in 1980. By replacing the hydroxy group, it was rationalised that chloramphenicol resistance via chloramphenicol acetyltransferase could be eliminated. Florfenicol is a broad spectrum antibiotic with good activity against Gram negative and anaerobic bacteria. It acts by binding to the 23S sub-unit of the 50S ribosome, inhibiting protein synthesis. Florfenicol has been extensively studied with over 400 literature citations.
Veterinary Drugs and Treatments
The drug is approved for use in cattle only (in the USA) for the
treatment of bovine respiratory disease (BRD) associated with
Pasteurella haemolytica, Pasteurella multocida, and Haemophilus
somnus.
Because florfenicol has activity against a wide range of microorganisms
(e.g., Mycoplasma), it may be useful for treating other
infections in cattle (or other species) as well, but specific data is
limited.
Properties of Florfenicol
| Melting point: | 153 °C |
| Boiling point: | 618℃ |
| alpha | 26 +17.9° (DMF) |
| Density | 1.451±0.06 g/cm3(Predicted) |
| RTECS | DB6034000 |
| Flash point: | >110°(230°F) |
| storage temp. | Inert atmosphere,2-8°C |
| solubility | Soluble in ethanol to 25mM and in DMSO to 100mM |
| form | solid |
| pka | 10.73±0.46(Predicted) |
| color | White to Off-White |
| Merck | 14,4109 |
| InChI | InChI=1S/C12H14Cl2FNO4S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-15)16-12(18)11(13)14/h2-5,9-11,17H,6H2,1H3,(H,16,18)/t9-,10-/m1/s1 |
| CAS DataBase Reference | 73231-34-2(CAS DataBase Reference) |
| EPA Substance Registry System | Florfenicol (73231-34-2) |
Safety information for Florfenicol
Computed Descriptors for Florfenicol
| InChIKey | AYIRNRDRBQJXIF-NXEZZACHSA-N |
| SMILES | C(N[C@H](CF)[C@H](O)C1=CC=C(S(C)(=O)=O)C=C1)(=O)C(Cl)Cl |
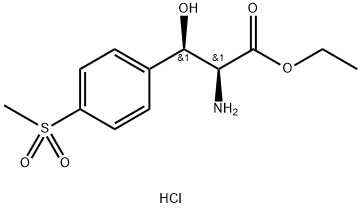
Related products of tetrahydrofuran
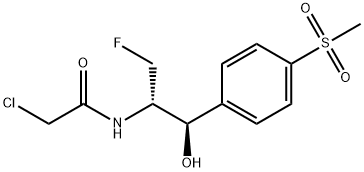
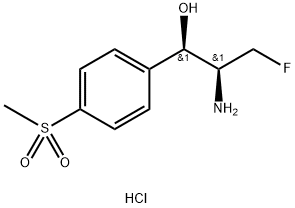
![2,2-dichloro-N-[(1R)-3-chloro-1-hydroxy-1-(4-methylsulfonylphenyl)propan-2-yl]acetamide](https://img.chemicalbook.in/CAS/20180601/GIF/1322625-10-4.gif)
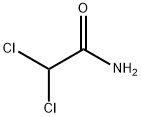
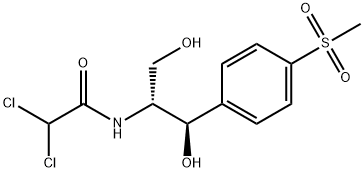
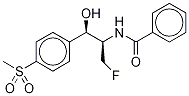
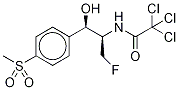
You may like
-
 Florfenicol 98% CAS 73231-34-2View Details
Florfenicol 98% CAS 73231-34-2View Details
73231-34-2 -
 Florfenicol CAS 73231-34-2View Details
Florfenicol CAS 73231-34-2View Details
73231-34-2 -
 Florfenicol CAS 73231-34-2View Details
Florfenicol CAS 73231-34-2View Details
73231-34-2 -
 Florfenicol CAS 73231-34-2View Details
Florfenicol CAS 73231-34-2View Details
73231-34-2 -
 37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 3'-Benzyloxy propiophenone, 98% 99%View Details
37951-47-6 -
 104944-18-5 99%View Details
104944-18-5 99%View Details
104944-18-5 -
 3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
3'-Methoxypropiophenone, 99% 37951-49-8 99%View Details
37951-49-8 -
 51364-51-3 99%View Details
51364-51-3 99%View Details
51364-51-3