ETHYLMERCURIC CHLORIDE
- CAS NO.:107-27-7
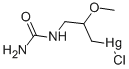
- Empirical Formula: C2H5ClHg
- Molecular Weight: 265.1
- MDL number: MFCD00013593
- EINECS: 203-478-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
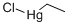
What is ETHYLMERCURIC CHLORIDE?
Chemical properties
silver crystals or white solid
Chemical properties
Ethyl methacrylate is a colorless liquid.
Chemical properties
Ethyl mercuric chloride, an alkyl-organo mercury compound, is silvery white, forming leaf-like crystals
The Uses of ETHYLMERCURIC CHLORIDE
Applied at 2% strength (soln or mixed with solids) as a fungicide for treating seeds.
The Uses of ETHYLMERCURIC CHLORIDE
Ethyl mercuric chloride is used as the polymerization catalyst; seed or bulb fungicide.
Definition
ChEBI: Ethylmercuric chloride is a highly toxic organomercury compound which is used as a fungicide for treating seeds. It has a role as a fungicide. It is a chlorine molecular entity and an organomercury compound.
General Description
Silver iridescent crystals or white fluffy solid . Sublimes easily. Sensitive to light. Highly toxic. Causes skin burns and is absorbed through the skin.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
ETHYLMERCURIC CHLORIDE is sensitive to prolonged exposure to light. Incompatible with strong oxidizers such as chlorine .
Hazard
Strong irritant.
Fire Hazard
Flash point data for ETHYLMERCURIC CHLORIDE are not available; however, ETHYLMERCURIC CHLORIDE is probably combustible.
Safety Profile
Poison by ingestion, inhalation, skin contact, and intraperitoneal routes. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. See also MERCURY COMPOUNDS, ORGANIC. When heated to decomposition it emits very toxic fumes of Cland Hg.
Potential Exposure
This chemical is used in manufacture of coatings, resins, and lacquers. Widely known as “Plexiglass” (in the polymer form), ethyl methacrylate is used to make polymers, which in turn are used for building, automotive, aerospace, and furniture industries. It is also used by dentists as dental plates, artificial teeth, and orthopedic cement.
Shipping
UN2277 Ethyl methacrylate, stabilized, Hazard Class: 3; Labels: 3-Flammable liquid
Purification Methods
Mercuric chloride can be removed by suspending ethylmercuric chloride in hot distilled water, filtering with suction onto a sintered-glass crucible and drying it. Then crystallise it from ethanol and sublime it under reduced pressure. It can also be crystallised from water. [Marvel et al. J Am Chem Soc 47 3009 1925.]
Incompatibilities
May form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine,etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides. Corrodes some metals. Unless inhibited, violent polymerization can occur from heat, sunlight, and contact with strong oxidizers
Waste Disposal
In accordance with 40CFR 165 recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of ETHYLMERCURIC CHLORIDE
| Melting point: | 195°C |
| Density | 3.482 |
| solubility | Chloroform (Slightly) |
| form | solid |
| Water Solubility | Insoluble in water. Slightly soluble in ether. Soluble in hot alcohol. Sublimes readilyInsoluble in water. Slightly soluble in ether. Soluble in hot alcohol. |
| Merck | 14,3826 |
| Exposure limits | NIOSH: IDLH 10 mg/m3; TWA 0.05 mg/m3; Ceiling 0.1 mg/m3 |
| Stability: | Stable. Incompatible with strong oxidizing agents. May be light sensitive. |
| CAS DataBase Reference | 107-27-7(CAS DataBase Reference) |
| EPA Substance Registry System | Mercury, chloroethyl- (107-27-7) |
Safety information for ETHYLMERCURIC CHLORIDE
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H310:Acute toxicity,dermal H330:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P320:Specific treatment is urgent (see … on this label). P330:Rinse mouth. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for ETHYLMERCURIC CHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
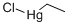
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1