Ethylene glycol monoethyl ether acetate
Synonym(s):1-Acetoxy-2-ethoxyethane;2-Ethoxyethyl acetate;Cellosolve acetate;Ethylene glycol monoethyl ether acetate;Ethylene glycol monoethyl ether acetate, Ethyl glycol acetate, Acetic acid 2-ethoxyethyl ester
- CAS NO.:111-15-9
- Empirical Formula: C6H12O3
- Molecular Weight: 132.16
- MDL number: MFCD00009251
- EINECS: 203-839-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:04
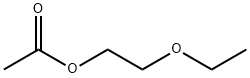
What is Ethylene glycol monoethyl ether acetate?
Description
2-Ethoxyethyl acetate is a colorless liquid witha mild, nonresidual odor. The odor threshold is 0.056 ppmin air. Molecular weight = 132.18; Specific gravity(H2O:1) = 0.98 at 20℃; Boiling point = 156℃; Freezing/Melting point = - 61.7℃; Vapor pressure = 2 mmHg at20℃; Flash point = 47℃; Autoignitiontemperature = 380℃. Explosive limits: LEL = 1.7%;UEL = 12.7%. The UEL is also reported as 14%. HazardIdentification (based on NFPA-704 M Rating System):Health 2, Flammability 2, Reactivity 0. Soluble in water;solubility = 23%
Chemical properties
colourless liquid
Chemical properties
2-Ethoxyethyl acetate is a colorless liquid with a mild, nonresidual odor. The Odor Threshold is 0.056 ppm in air
Physical properties
Colorless liquid with a faint, pleasant odor. Experimentally determined detection and recognition odor threshold concentrations were 300 μg/m3 (60 ppbv) and 700 μg/m3 (130 ppbv), respectively (Hellman and Small, 1974). Nagata and Takeuchi (1990) reported an odor threshold concentration of 49 ppbv.
The Uses of Ethylene glycol monoethyl ether acetate
Solvent for nitrocellulose, oils, and resins; retards “blushing” in lacquers, varnish removers, wood stains, textiles, and leather.
The Uses of Ethylene glycol monoethyl ether acetate
In the coatings industry, especially in the semiconductor industry; solvent for nitrocellulose and some resins
The Uses of Ethylene glycol monoethyl ether acetate
2-Ethoxyethyl acetate is used as a solvent dissolve resin, leather, ink, for the formulation of paints, lacquers and varnishes for industrial use. It is also used to study the field evaluation of a passive badge for measuring 2-ethoxyethyl acetate.
General Description
A clear colorless liquid with a pleasant odor. Flash point of 120°F. Less dense than water. Vapors are heavier than air.
Air & Water Reactions
Flammable. Slightly soluble in water.
Reactivity Profile
Mixing Ethylene glycol monoethyl ether acetate in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, oleum, and vinyl acetate, NFPA 1991.
Hazard
Toxic by ingestion and skin absorption. Toxic by skin absorption.
Health Hazard
Vapors irritate nose and eyes in high concentrations. Liquid irritates skin in prolonged or repeated contact.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. A skin and eye irritant. An experimental teratogen. Other experimental reproductive effects. Flammable liquid when exposed to heat or flame; can react with oxidizing materials. Moderate explosion hazard in the form of vapor when heated. Mild explosions have occurred at the end of disullations. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS.
Potential Exposure
This material is used as a solvent for many different purposes; including for nitrocellulose and other resins. Used in automobile lacquers to retard evaporation and impart a high gloss.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Biological. Bridié et al. (1979) reported BOD and COD values of 0.74 and 1.76 g/g using
filtered effluent from a biological sanitary waste treatment plant. These values were determined
using a standard dilution method at 20 °C for a period of 5 d. The ThOD for 2-ethoxyethyl acetate
is 1.82 g/g.
Chemical/Physical. At an influent concentration of 1,000 mg/L, treatment with GAC resulted
in an effluent concentration of 342 mg/L. The adsorbability of the carbon used was 132 mg/g
carbon (Guisti et al., 1974).
Storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist. 2-Ethoxyethyl acetate must be stored to avoid contact withstrong oxidizers, such as nitrates, permanganates, bromine,chlorine, and chlorine dioxide; strong alkalis, such assodium hydroxide and potassium hydroxide; and strongacids, such as nitric, hydrochloric, and sulfuric acids, sinceviolent reactions occur. Store in tightly closed containers ina cool, well-ventilated area away from heat. Sources ofignition, such as smoking and open flames, are prohibitedwhere 2-ethoxyethyl acetate is used, handled, or stored in amanner that could create a potential fire or explosionhazard.
Shipping
UN1172 Ethylene glycol monoethyl ether acetate, Hazard Class: 3; Labels: 3-Flammable liquid
Purification Methods
Shake the ethoxy-ethane with anhydrous Na2CO3, filter and distil it in a vacuum. Redistillation can then be carried out at atmospheric pressure. [Dunbar & Bolstad J Org Chem 21 1041 1956, Beilstein 2 IV 214.]
Incompatibilities
May form explosive mixture with air. Incompatible with strong acids; strong alkalies; nitrates. Violent reaction with oxidizers. May form unstable peroxides. Softens many plastics. Attacks some plastics, rubber, and coatings
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Ethylene glycol monoethyl ether acetate
| Melting point: | -61 °C |
| Boiling point: | 156 °C(lit.) |
| Density | 0.975 g/mL at 25 °C(lit.) |
| vapor density | 4.6 (vs air) |
| vapor pressure | 2 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 135 °F |
| storage temp. | Store below +30°C. |
| solubility | H2O: solubleabout 6 parts |
| form | Colorless liquid |
| color | Colorless |
| PH | 4-5 (H2O, 20℃)(saturated solution) |
| explosive limit | 1.7-10%(V) |
| Odor Threshold | 0.049ppm |
| Water Solubility | 230 g/L (20 ºC) |
| Merck | 14,3751 |
| BRN | 1748677 |
| Henry's Law Constant | 9.07(x 10-7 atm?m3/mol) at 25 °C (approximate - calculated from water solubility and vapor pressure)
33.0(x 10-7 atm?m3/mol) at 30.00 °C (headspace-GC, Hovorka et al., 2002) |
| Exposure limits | NIOSH REL: TWA 0.5 ppm (2.7 mg/m3), IDLH 500 ppm; OSHA PEL: TWA
100 ppm (540 mg/m3); ACGIH TLV: TWA 5 ppm (adopted). |
| Dielectric constant | 7.6(30℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, strong acids, strong bases, nitrates. |
| CAS DataBase Reference | 111-15-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Ethoxyethyl acetate(111-15-9) |
| EPA Substance Registry System | Ethylene glycol monoethyl ether acetate (111-15-9) |
Safety information for Ethylene glycol monoethyl ether acetate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H226:Flammable liquids |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Ethylene glycol monoethyl ether acetate
Ethylene glycol monoethyl ether acetate manufacturer
Oswal Udhyog
R J Organics
Techno Pharmchem
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 2-Ethoxy ethyl acetate, puriss CAS 111-15-9View Details
2-Ethoxy ethyl acetate, puriss CAS 111-15-9View Details
111-15-9 -
 2-Ethoxyethyl Acetate CAS 111-15-9View Details
2-Ethoxyethyl Acetate CAS 111-15-9View Details
111-15-9 -
 2-Ethoxy ethyl acetate CAS 111-15-9View Details
2-Ethoxy ethyl acetate CAS 111-15-9View Details
111-15-9 -
 CELLOSOLVE ACETATE For Synthesis CAS 111-15-9View Details
CELLOSOLVE ACETATE For Synthesis CAS 111-15-9View Details
111-15-9 -
 Cellosolve acetate 99% CAS 111-15-9View Details
Cellosolve acetate 99% CAS 111-15-9View Details
111-15-9 -
 Ethyl Cellosolve Acetate CASView Details
Ethyl Cellosolve Acetate CASView Details -
 Cellosolve Acetate CAS: 111-15-9View Details
Cellosolve Acetate CAS: 111-15-9View Details
111-15-9 -
 Ethyl Cellosolve AcetateView Details
Ethyl Cellosolve AcetateView Details
111-15-9
