Nickel carbonate
- CAS NO.:3333-67-3
- Empirical Formula: CNiO3
- Molecular Weight: 118.7
- MDL number: MFCD00011144
- EINECS: 222-068-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:11:47
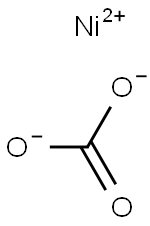
What is Nickel carbonate?
Chemical properties
Nickel carbonate is a light green crystalline substance, which is almost insoluble (0.093 g/L) in water (25°C), nonsoluble in hot water, and soluble in acids. Nickel carbonate is available primarily as basic nickel carbonate (NiCO3· 2Ni(OH)2 · 4H2O), which is not soluble in water and soluble in ammonia and dilute acids. In the natural environment, nickel carbonate tetrahydrate can be found as zaratite.

Physical properties
NiCO3: Light green rhombohedral crystals; decomposes on heating; practically insoluble in water, 93 mg/L at 25°C; dissolves in acids.
2NiCO3?3Ni(OH)2?4H2O: Light green crystals or brown powder; decomposes on heating; insoluble in water; decomposes in hot water; soluble in acids and in ammonium salts solutions.
Zaratite: Emerald greed cubic crystals; density 2.6 g/cm3; insoluble in water; soluble in ammonia and dilute acids.
The Uses of Nickel carbonate
Nickel carbonate is used to prepare nickel catalysts and several specialty compounds of nickel. It also is used as a neutralizing agent in nickel plating solutions. Other applications are in coloring glass and in the manufacture of ceramic pigments.
The Uses of Nickel carbonate
Nickel(II) carbonate is used in sulphamate baths, metal phosphating, electroplating and ceramic applications. It acts as a precursor to catalysts and an intermediate in the hydrometallurgical purification of nickel from its ores.
Preparation
Anhydrous nickel carbonate is produced as a precipitate when calcium carbonate is heated with a solution of nickel chloride in a sealed tube at 150°C. Alternatively, treating nickel powder with ammonia and carbon dioxide followed by boiling off ammonia yields pure carbonate.
When sodium carbonate is added to a solution of Ni(II) salts, basic nickel carbonate precipitates out in impure form.
Reactions
Nickel carbonate is the starting material for preparing many nickel salts. It reacts with dilute acids evolving carbon dioxide, and upon evaporation of the solution corresponding nickel salts are formed. The nitrate, sulfate and phosphate salts are prepared from carbonate. Similarly, reactions with hydrofluoric, hydrochloric, hydrobromic, or hydriodic acids yield hydrated nickel halides: namely NiF2•4H2O, NiCl2•6H2O, NiBr2•6H2O, and NiI2•6H2O, respectively:
NiCO3 + HCl → NiCl2•6H2O + CO2
NICKEL CARBONATE 611Nickel carbonate decomposes to nickel oxide when strongly ignited:
NiCO3 → NiO + CO2
Nickel carbonate, when dissolved in aqueous thiocyanic acid, yields a yellow brown precipitate of hydrated nickel thiocyanate:
2 NiCO3 + 2HSCN → Ni(SCN)2 + CO2 + H2O
Nickel carbonate forms many double salts, such as, Na2CO3•NiCO3•10H2O with alkali metal carbonates. However, such double carbonates usually are prepared by mixing an alkali metal or ammonium bicarbonate solution with a nickel salt solution, followed by crystallization.
Hazard
Confirmed carcinogen.
Safety Profile
Confirmed human carcinogen. When heated to decomposition it emits toxic vapors of nickel.
Properties of Nickel carbonate
| Melting point: | decomposes [CRC10] |
| Density | 4.390 |
| solubility | soluble in dilute acid solutions |
| form | Solid |
| color | Light Green |
| Water Solubility | Soluble in dilute acids. Insoluble in cold water. |
| Solubility Product Constant (Ksp) | pKsp: 6.85 |
| Exposure limits | ACGIH: TWA 0.2 mg/m3 NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability: | Stable. Incompatible with strong acids. |
| CAS DataBase Reference | 3333-67-3(CAS DataBase Reference) |
| EPA Substance Registry System | Nickel carbonate (3333-67-3) |
Safety information for Nickel carbonate
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H332:Acute toxicity,inhalation H334:Sensitisation, respiratory H341:Germ cell mutagenicity H350:Carcinogenicity H360:Reproductive toxicity H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P405:Store locked up. |
Computed Descriptors for Nickel carbonate
Nickel carbonate manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 NICKEL CARBONATE 99%View Details
NICKEL CARBONATE 99%View Details -
 Nickel(II) carbonate 99%View Details
Nickel(II) carbonate 99%View Details -
 3333-67-3 98%View Details
3333-67-3 98%View Details
3333-67-3 -
 Nickel(II) carbonate 3333-67-3 98%View Details
Nickel(II) carbonate 3333-67-3 98%View Details
3333-67-3 -
 3333-67-3 98%View Details
3333-67-3 98%View Details
3333-67-3 -
 Nickel(II) carbonate, anhydrous CAS 3333-67-3View Details
Nickel(II) carbonate, anhydrous CAS 3333-67-3View Details
3333-67-3 -
 Nickel(II) carbonate, anhydrous CAS 3333-67-3View Details
Nickel(II) carbonate, anhydrous CAS 3333-67-3View Details
3333-67-3 -
 Nickel(II) carbonate CAS 3333-67-3View Details
Nickel(II) carbonate CAS 3333-67-3View Details
3333-67-3
