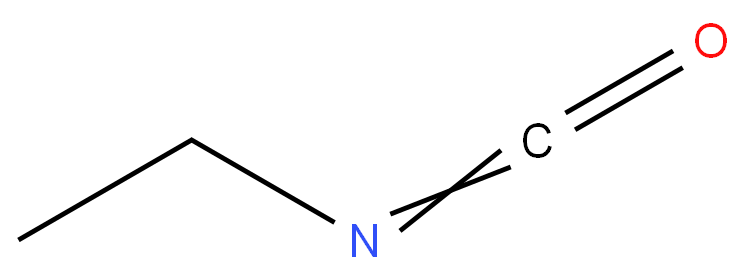
Ethyl isocyanate
- CAS NO.:109-90-0
- Empirical Formula: C3H5NO
- Molecular Weight: 71.08
- MDL number: MFCD00002042
- EINECS: 203-717-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
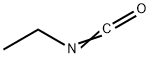
What is Ethyl isocyanate?
Chemical properties
Ethyl Isocyanate is a clear, colorless liquid with a pungent odor.
The Uses of Ethyl isocyanate
Ethyl isocyanate is part of a group of isocyanates that react with primary and secondary amines to form urea derivatives and carbamates. Ethyl isocyanate (among other isocyanate compounds, such as toluene isocyanate) are also thought to be the most common cause of occupational asthma.
The Uses of Ethyl isocyanate
Pharmaceutical and pesticide intermediate.
Definition
ChEBI: An isocyanate having an ethyl group attached to the nitrogen.
General Description
A colorless liquid. Less dense than water and insoluble in water. Flash point below 30°F. May irritate skin, eyes, and mucous membranes. May be lethal by Inhalation . Used to make pharmaceuticals and pesticides.
Air & Water Reactions
Highly flammable. Insoluble in water. Ethyl isocyanate may react with water to produce a corrosive liquid and carbon dioxide gas.
Reactivity Profile
When heated to decomposition Ethyl isocyanate emits toxic fumes of nitrogen oxides [Sax, 9th ed., 1996, p. 1572].
Hazard
Strong irritant to tissue.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Bromoacetates and chloroacetates are extremely irritating/lachrymators. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapors may travel to source of ignition and flash back. Substance will react with water (some violently) releasing flammable, toxic or corrosive gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
Poison by intravenous route. Mutation data reported. A flammable liquid. When heated to decomposition it emits toxic fumes of NOx. See also CYANATES.
Potential Exposure
Ethyl isocyanate is used to make pharmaceuticals and pesticides
Shipping
UN2481 Ethyl isocyanate, Hazard class: 6.1; Labels: 6.1-Poison Inhalation Hazard, 3-Flammable liquid, Inhalation Hazard Zone A. Cylinders must be transported in a secure upright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.
Purification Methods
Fractionate the isocyanate through an efficient column preferably in an inert atmosphere and store it in aliquots in sealed tubes [Bieber J Am Chem Soc 74 4700 1952, Slocombe et al. J Am Chem Soc 72 1888 1950]. [Beilstein 4 IV 402.]
Incompatibilities
Vapor may form explosive mixture with air. May form explosive mixture with air. Isocyanates are highly flammable and reactive with many compounds, even with themselves. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Reaction with moist air, water or alcohols may form amines and insoluble polyureas and react exothermically, releasing toxic, corrosive or flammable gases, including carbon dioxide; and, at the same time, may generate a violent release of heat increasing the concentration of fumes in the air. Incompatible with amines, aldehydes, alkali metals, ammonia, carboxylic acids, caprolactum, alkaline materials, glycols, ketones, mercaptans, hydrides, organotin catalysts, phenols, strong acids, strong bases, strong reducing agents such as hydrides, urethanes, and ureas. Elevated temperatures or contact with acids, bases, tertiary amines, and acyl-chlorides may cause explosive polymerization. Contact Attacks some plastics, rubber and coatings. Contact with metals may evolve flammable hydrogen gas. May accumulate static electrical charges, and may cause ignition of its vapors.
Properties of Ethyl isocyanate
| Melting point: | <-50 |
| Boiling point: | 60 °C(lit.) |
| Density | 0.898 g/mL at 25 °C(lit.) |
| vapor pressure | 4.34 psi ( 20 °C) |
| refractive index | n |
| Flash point: | 20 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Oil |
| color | Colourless |
| Water Solubility | Insoluble |
| Sensitive | Moisture Sensitive |
| BRN | 773743 |
| Stability: | Hygroscopic, Moisture sensitive, Volatile |
| CAS DataBase Reference | 109-90-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethane, isocyanato-(109-90-0) |
| EPA Substance Registry System | Ethyl isocyanate (109-90-0) |
Safety information for Ethyl isocyanate
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H225:Flammable liquids H301:Acute toxicity,oral H315:Skin corrosion/irritation H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Ethyl isocyanate
Ethyl isocyanate manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran

You may like
-
 109-90-0 isocyanatoethane 98%View Details
109-90-0 isocyanatoethane 98%View Details
109-90-0 -
 109-90-0 98%View Details
109-90-0 98%View Details
109-90-0 -
 109-90-0 98%View Details
109-90-0 98%View Details
109-90-0 -
 Ethyl-2-Chloroacetoacetate 609-15-4View Details
Ethyl-2-Chloroacetoacetate 609-15-4View Details
609-15-4 -
 CIS- BROMO BENZOATEView Details
CIS- BROMO BENZOATEView Details
61397-56-6 -
 609-15-4View Details
609-15-4View Details
609-15-4 -
![1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%](https://img.chemicalbook.in//Content/image/CP5.jpg) 1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
1-(6-Methylpyridin-3-Yl)-2-[4-(Methylsulfonyl)Phenyl]Ethanone [Ketosulfone] 99%View Details
221615-75-4 -
 27143-07-3View Details
27143-07-3View Details
27143-07-3
