Ethyl acetoacetate
Synonym(s):Acetoacetic ester;EAA;EAA, Acetoacetic acid ethyl ester;Ethyl 3-oxobutanoate;Ethyl acetoacetate
- CAS NO.:141-97-9
- Empirical Formula: C6H10O3
- Molecular Weight: 130.14
- MDL number: MFCD00009199
- EINECS: 205-516-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-03-18 22:05:36
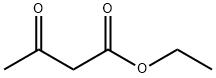
What is Ethyl acetoacetate?
Description
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is mainly used as a chemical intermediate in the production of a wide variety of compounds, such as amino acids, analgesics, antibiotics, antimalarial agents, antipyrine and amino pyrine, and vitamin B1; as well as the manufacture of dyes, inks, lacquers, perfumes, plastics, and yellow paint pigments. Alone, it is used as a flavoring for food.
Chemical properties
Ethyl acetoacetate is a colorless liquid with a fruity, ethereal, sweet odor reminiscent of green apples. It is used to create fresh, fruity top notes in feminine fine fragrances. Ethyl acetoacetate occurs in flavors of natural materials such as coffee, strawberries, and yellow passion fruits.
Occurrence
Naturally occurring in strawberry, coffee, sherry, passion fruit juice (yellow), babaco fruit (Carica pentagona Heilborn) and bread.
The Uses of Ethyl acetoacetate
Ethyl acetoacetate is used as an intermediate in organic synthesis and as a co-promoter for unsaturated polyester resins. It is widely used in the production of dyes, inks, perfumes, plastics and flavoring agents. It is an important starting material for the syntheses of alpha-substituted acetoacetic esters and cyclic compounds like pyrazole, pyrimidine and coumarin derivatives. It acts as an intermediate in the synthesis of vitamins and pharmaceuticals. It finds application as a formaldehyde scavenger.
Definition
This compound is a tautomer at room temperature consisting of about 93% keto form and 7% enol form.
Preparation
Ethyl acetoacetate is produced industrially by treatment of diketene with ethanol. The preparation of ethyl acetoacetate is a classic laboratory procedure. It is prepared via the Claisen condensation of ethyl acetate. It is manufactured through a reaction of high-purity ethyl acetate with sodium, followed by neutralization with sulfuric acid. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol.
Aroma threshold values
Detection: 520 ppb. Aroma characteristics at 10%: sweet fruity apple, fermented, slightly fusel-like and rummy, fruity banana with tropical nuances.
Taste threshold values
Taste characteristics at 100 ppm: fruity banana, apple and white grape with slightly green estry and tropical nuances.Taste characteristics at 300 ppm: estery, fatty, fruity and tutti-frutti
Synthesis Reference(s)
The Journal of Organic Chemistry, 58, p. 793, 1993 DOI: 10.1021/jo00055a046
General Description
A colorless liquid with a fruity odor. Flash point 185°F. Boiling point 365°F. May cause adverse health effects if ingested or inhaled. May irritate to skin, eyes and mucous membranes. Used in organic synthesis and in lacquers and paints.
Air & Water Reactions
Flammable.
Reactivity Profile
Ethyl acetoacetate, a beta-keto ester, is more reactive than many esters. Undergoes an exothermic cleavage reaction in the presence of concentrated base. Reacts with acids to liberate heat along with alcohols and acids. Strong oxidizing acids may cause a vigorous reaction that is sufficiently exothermic to ignite the reaction products. Flammable hydrogen is generated by mixing esters with alkali metals and hydrides. Mixing with 2,2,2-tris(bromomethyl)ethanol and zinc led to an explosion [US Patent 3 578 619, Crotonaldehyde may rapidly polymerize with Ethyl acetoacetate (Soriano, D.S. et al. 1988. Journal of Chemical Education 65:637.).1971].
Hazard
Toxic by ingestion and inhalation; irritant to skin and eyes.
Health Hazard
Liquid may cause mild irritation of eyes.
Flammability and Explosibility
Non flammable
Chemical Reactivity
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Chemical Reactivity
Ethyl acetoacetate is subject to Keto - enol tautomerism. Ethyl acetoacetate is often used in the acetoacetic ester synthesis similar to diethyl malonate in the malonic ester synthesis or the Knoevenagel condensation. The protons alpha to carbonyl groups are acidic, and the resulting carbanion can undergo nucleophilic substitution. A subsequent thermal decarboxylation is also possible.Similar to the behavior of acetylacetone, the enolate of ethyl acetoacetate can also serve as a bidentate ligand. For example, it forms purple coordination complexes with iron (III) salts :
Ethyl acetoacetate can also be reduced to ethyl 3-hydroxy butyrate.
Safety Profile
eye irritant. Combustible liquid when exposed to heat or flame; can react with oxidzing materials. Explosive reaction when heated with Zn + tribromoneopentyl alcohol or 2,2,2 tris(bromomethy1)ethanol. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTERS.
Synthesis
Ethyl acetoacetate is a mixture of two tautomer forms: the enolic and the ketonic; the liquid ester at equilibrium contains approximately 70% of the enolic form. It is prepared by Claisen condensation of ethyl acetate in the presence of sodium ethylate; also by reacting diketene with ethanol in the presence of sulfuric acid or triethylamine and sodium acetate, with or without solvent.
Purification Methods
Shake the ester with small amounts of saturated aqueous NaHCO3 (until no further effervescence), then with water. Dry it with MgSO4 or CaCl2 and distil it under reduced pressure. [Beilstein 3 IV 1528.]
Properties of Ethyl acetoacetate
| Melting point: | −43 °C(lit.) |
| Boiling point: | 181 °C(lit.) |
| Density | 1.029 g/mL at 20 °C(lit.) |
| vapor density | 4.48 (vs air) |
| vapor pressure | 1 mm Hg ( 28.5 °C) |
| refractive index | n |
| FEMA | 2415 | ETHYL ACETOACETATE |
| Flash point: | 185 °F |
| storage temp. | Store below +30°C. |
| solubility | 116 g/L (20°C) |
| form | Liquid |
| pka | 11(at 25℃) |
| Specific Gravity | 1.027~1.035 (20/4℃) |
| color | APHA: ≤15 |
| Odor | Agreeable, fruity. |
| Relative polarity | 0.577 |
| PH | 4.0 (110g/l, H2O, 20℃) |
| explosive limit | 1.0-54%(V) |
| Water Solubility | 116 g/L (20 ºC) |
| Merck | 14,3758 |
| JECFA Number | 595 |
| BRN | 385838 |
| Dielectric constant | 15.0(Ambient) |
| Stability: | Stable. Incompatible with acids, bases, oxidizing agents, reducing agents, alkali metals. Combustible. |
| CAS DataBase Reference | 141-97-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Butanoic acid, 3-oxo-, ethyl ester(141-97-9) |
| EPA Substance Registry System | Ethyl acetoacetate (141-97-9) |
Safety information for Ethyl acetoacetate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids H303:Acute toxicity,oral H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P337+P313:IF eye irritation persists: Get medical advice/attention. P370+P378:In case of fire: Use … for extinction. P403+P235:Store in a well-ventilated place. Keep cool. P501:Dispose of contents/container to..… |
Computed Descriptors for Ethyl acetoacetate
| InChIKey | XYIBRDXRRQCHLP-UHFFFAOYSA-N |
Ethyl acetoacetate manufacturer
New Products
Methyl (R)-1-Boc-4,4-difluoropyrrolidine-2-carboxylate 2,2-Difluoropropylamine hydrochloride tert-butyl 3-bromoazetidine-1-carboxylate (R)-1-Boc-3-hydroxypyrrolidine DIFLUOROACETIC ANHYDRIDE 2,2-Difluoropropionic acid Diallylamine, 99% Calcium hydroxide, 95% Aluminum oxide, basic 2-Bromophenylacetonitrile, 97% L-tert-Leucine,97% N-Hydroxy-2-methylpropanimidamide 4-(3,4-Dichlorophenyl)-3,4-Dihydro-N-Methyl-1-(2H)-Naphthalenimine (Schiff Base) 2-AMINO-3,5-DIBROMO BENZALDEHYDE [ADBA] L-Glutamic Acid Dimethyl Ester Hcl 10-Methoxy-5H-dibenz[b,f]azepine 5-Cyanophthalide N, N-Carbonyldiimidazole (CDI) Dibenzoyl Peroxide Titanium Dioxide 2-(Methylthio) Benzonitrile Sodium Acetate Anhydrous Allopurinol 1,5-DibromopentaneRelated products of tetrahydrofuran
You may like
-
 141-97-9 98%View Details
141-97-9 98%View Details
141-97-9 -
 Ethyl Aceto Acetate 99%View Details
Ethyl Aceto Acetate 99%View Details -
 Ethyl acetoacetate CAS 141-97-9View Details
Ethyl acetoacetate CAS 141-97-9View Details
141-97-9 -
 Ethyl aceto acetate CAS 141-97-9View Details
Ethyl aceto acetate CAS 141-97-9View Details
141-97-9 -
 Ethyl Acetoacetate CAS 141-97-9View Details
Ethyl Acetoacetate CAS 141-97-9View Details
141-97-9 -
 Ethyl Acetoacetate CASView Details
Ethyl Acetoacetate CASView Details -
 Ethyl aceto acetate, GR 99%+ CAS 141-97-9View Details
Ethyl aceto acetate, GR 99%+ CAS 141-97-9View Details
141-97-9 -
 Ethyl Aceto Acetate CASView Details
Ethyl Aceto Acetate CASView Details
