ETHYL 2-CYANOACRYLATE
- CAS NO.:7085-85-0
- Empirical Formula: C6H7NO2
- Molecular Weight: 125.13
- MDL number: MFCD00045615
- EINECS: 230-391-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41
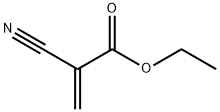
What is ETHYL 2-CYANOACRYLATE?
Description
Ethyl cyanoacrylate is contained in instant glues for metal, glass, rubber, plastics, textiles, tissues and nails. It polymerizes almost instantaneously in air at room temperature and bonds immediately and strongly to surface keratin. Beauticians are exposed to contact dermatitis from nail glues.
Description
Ethyl 2-cyanoacrylate is a liquid that you’ve probably used around the house. Because of its conjugated unsaturated groups, it polymerizes easily. It is prepared by condensing ethyl 2-cyanoacetate with formaldehyde, followed by thermal decomposition of the resulting polymer.
Ethyl 2-cyanoacrylate and other less common 2-cyanoacrylate esters are the active ingredients in commercial superglues or instant glues. Some of these products contain additives such as fumed silica to make them more viscous or rubber to make them more impact resistant.
Cyanoacrylates were discovered serendipitously during World War II when scientists at B.F. Goodrich (located in Akron, OH at the time) sought suitable clear plastics for gun sights. Chemist Harry Coover Jr. and co-workers found that the esters auto-polymerized and stuck to everything in sight. Because of the wartime urgency for other materials, cyanoacrylates were abandoned until 1951, when Coover, then at Eastman Chemical (Eastport, TN), and colleague Fred Joyner “rediscovered” it. The first commercial glue appeared in 1958.
Ethyl 2-cyanoacrylate and its cousins polymerize instantly in water. The small amount of moisture in air is enough to initiate polymerization. The polymer forms within a matter of minutes, creating a bond that is often stronger than the materials it joins.
Despite its strength, the polymer can be removed by soaking it in acetone or mild acids. Household products such as nail polish remover or lemon juice will do the trick.
Chemical properties
Clear Colourless Liquid
The Uses of ETHYL 2-CYANOACRYLATE
A cosmetic composition
The Uses of ETHYL 2-CYANOACRYLATE
Ethyl cyanoacrylate is an acrylate compound used in instant glues to mend broken nails and to adhere glue-impregnated silk or linen to the nail plate, which is then filed to shape the nail; in medicine to glue tissues and skin cracks; to attach hair and to glue shoes, plastics, and many other materials.
The Uses of ETHYL 2-CYANOACRYLATE
ECA can be used as a tissue adhesive for potential applications in cardiovascular and pulmonary surgeries. It can also be mixed with hydrophobic silica nanoparticles for the designing of cotton fabrics for medical applications.
What are the applications of Application
Ethyl 2-Cyanoacrylate is a composition for cosmetics
General Description
Ethyl 2-cyanoacrylate (ECA) is a monomeric adhesive that can be used on a variety of materials such as metals, plastics, rubber, ceramics, and wood. It forms a one part adhesive system that can also be used as a primer.
Hazard
An inhalation hazard.
Flammability and Explosibility
Not classified
Safety Profile
An inhalation hazard. When heated to decomposition it emits toxic vapors of NOx.
Properties of ETHYL 2-CYANOACRYLATE
| Melting point: | -20 to -25° |
| Boiling point: | bp 54-56° (0.21-0.40 kPa) |
| Density | 1.04 |
| vapor pressure | 21Pa at 20℃ |
| refractive index | nD20 1.4391 |
| Flash point: | 85 °C |
| storage temp. | 2-8°C |
| solubility | Acetontrle (Slightly), Benzene (Slightly), Chloroform (Slightly) |
| appearance | colorless liquid |
| form | liquid |
| color | Clear colorless to slightly yellow |
| Water Solubility | 24μg/L at 20℃ |
| Stability: | Unstable - polymerizes rapidly, especially in the presence of moisture. Combustible. Incompatible with strong oxidizing agents, moisture. |
| CAS DataBase Reference | 7085-85-0(CAS DataBase Reference) |
| EPA Substance Registry System | Ethyl 2-cyanoacrylate (7085-85-0) |
Safety information for ETHYL 2-CYANOACRYLATE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ETHYL 2-CYANOACRYLATE
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Ethyl 2-cyanoacrylate CAS 7085-85-0View Details
Ethyl 2-cyanoacrylate CAS 7085-85-0View Details
7085-85-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
