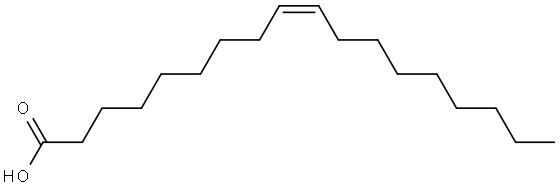
Erucic Acid
Synonym(s):cis-13-Docosenoic acid;cis-Erucic acid;Prifac 2990
- CAS NO.:112-86-7
- Empirical Formula: C22H42O2
- Molecular Weight: 338.57
- MDL number: MFCD00063188
- EINECS: 204-011-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-23 21:30:31

What is Erucic Acid?
Description
Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1ω9. It has the formula CH3(CH2)7CH=CH(CH2)11COOH. It is prevalent in wallflower seed, makes up 4.1% of rapeseed oil, and makes up 42% of mustard oil. Erucic acid is also known as cis-13- docosenoic acid and the trans isomer is known as brassidic acid.
Description
Erucic acid also known as cis-13-Docosenoic acid is a monounsaturated omega-9 fatty acid. It occurs at high concentrations mainly in the seeds of species of the Brassicaceae (e.g. rape seed or mustard seed, and seeds from vegetable crops such as kales, cabbages and turnips).
High-erucic acid oils are used either directly as lubricants (e.g. in the manufacture of rubber additives) or in formulations. They are used as spinning lubricants in the textile, steel, and shipping industries; as cutting, metal-forming, rolling, fabricating, and drilling oils; and as marine lubes. Erucic acid can also be oxidatively cleaved to brassylic acid for use in the production of polyesters. The oxidative cleavage of erucic acid can be performed via ozonolysis or by reaction with hydrogen peroxide in the presence of an inorganic oxide catalyst. Erucic acid can be used to prepare useful nitrogen derivatives: behenyl amine is used in a corrosion inhibitor; disubstituted amides are effective plasticizers and erucamide is an excellent slip and antiblocking agent for plastic films.
Chemical properties
Erucic acid is a monounsaturated omega-9 fatty acid, denoted 22:1ω9. It has the formula CH3(CH2)7CH=CH(CH2)11COOH. Colorless needle crystal. Melting point 33.5°C, boiling point 381.5°C (decomposition), 358°C (53.3kPa), 265°C (2.0kPa), relative density 0.86 (55°C), refractive index 1.4534 (45°C). Erucic acid is highly soluble in ether and soluble in ethanol and methanol, but it is insoluble in water. It is commonly found in wallflower seeds, accounting for 4.1% of rapeseed oil, and makes up 42% of mustard oil.
The Uses of Erucic Acid
Erucic acid has many of the same uses as mineral oils, but it is more readily biodegradable than some. It has limited ability to polymerize and dry for use in oil paints. Like other fatty acids, it can be converted into surfactants or lubricants, and can be used as a precursor to bio-diesel fuel.
Derivatives of erucic acid have many further uses, such as behenyl alcohol ( CH3(CH2)21OH ) , a pour point depressant (enabling liquids to flow at a lower temperature), and silver behenate, for use in photography. It is also used as an ingredient in appetite suppressants.
The Uses of Erucic Acid
13(Z)-Docosenoic acid is a 22-carbon monounsaturated fatty acid. It is found predominantly in canola oil. 13(Z)-Docosenoic acid is metabolized to oleic acid in vivo. Diets rich in 13(Z)-docosenoic acid were shown to cause heart lipidosis in experimental animals. The C-1 amide of docosenoic acid has been identified as one of the anandamide-related neurotransmitters associated with sleep.
What are the applications of Application
13(Z)-Docosenoic Acid is a 22-carbon monounsaturated fatty acid which is metabolized to oleic acid
What are the applications of Application
Erucic Acid is a monounsaturated fatty acid
Definition
ChEBI: Erucic acid is a docosenoic acid having a cis- double bond at C-13. It is found particularly in brassicas - it is a major component of mustard and rapeseed oils and is produced by broccoli, Brussels sprouts, kale, and wallflowers. It is a long-chain alcohol that acts as an inhibitor of fatty acid oxidation in the heart.
Biotechnological Applications
Erucic acid is produced by elongation of oleic acid via oleoylcoenzyme A and malonyl- CoA. Erucic acid is broken down into shorter- chain fatty acids in the human liver by the long - chain Acyl CoA dehydrogenase enzyme.
Purification Methods
Crystallise erucic acid from MeOH. [Beilstein 2 IV 1676.]
References
[1] J.M. Vargas-Lopez, D. Wiesenborna, K. Tostenson , L. Cihacek (1999) Processing of Crambe for Oil and Isolation of Erucic Acid, JAOCS, 76, 801-809 DOI:10.1007/S11746-999-0069-4
[2] H. J. Nieschlag, I. A. Wolff (1971) Industrial uses of high erucic oils, JAOCS, 48, 723-727 DOI:10.1007/BF02638529
[3] Erucic Acid (22:1n-9) in Fish Feed, Farmed, and Wild Fish and Seafood Products DOI:10.3390/nu10101443
[4] Erucic acid--an inhibitor of fatty acid oxidation in the heart. DOI:10.1016/0005-2760(72)90130-0
[5] Purification-of-Laboratory-Chemicals
[6] Christophersen, B. & Bremer, J.: BBA-Lipid. Lipid Met., 280, 506 (1972); Ecke, W., et al.: Theor. Appl. Gen., 91, 972 (1995); Metz, J., et al.: Plant Phys., 122, 635 (2000)
Properties of Erucic Acid
| Melting point: | 28-32 °C (lit.) |
| Boiling point: | 358 °C/400 mmHg (lit.) |
| Density | 0,86 g/cm3 |
| refractive index | nD45 1.4534; nD65 1.44794 |
| Flash point: | 265°C/15mm |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.78±0.10(Predicted) |
| color | White to Off-White Low-Melting |
| Merck | 14,3674 |
| BRN | 1728049 |
| CAS DataBase Reference | 112-86-7(CAS DataBase Reference) |
| NIST Chemistry Reference | Erucic acid(112-86-7) |
| EPA Substance Registry System | 13-Docosenoic acid, (13Z)- (112-86-7) |
Safety information for Erucic Acid
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Erucic Acid
Erucic Acid manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 112-86-7 99%View Details
112-86-7 99%View Details
112-86-7 -
 Erucic acid CAS 112-86-7View Details
Erucic acid CAS 112-86-7View Details
112-86-7 -
 Erucic Acid CAS 112-86-7View Details
Erucic Acid CAS 112-86-7View Details
112-86-7 -
 Erucic acid CAS 112-86-7View Details
Erucic acid CAS 112-86-7View Details
112-86-7 -
 Erucic acid CAS 112-86-7View Details
Erucic acid CAS 112-86-7View Details
112-86-7 -
 ERUCIC ACID 90%View Details
ERUCIC ACID 90%View Details
112-86-7 -
 Industrial Grade Erucic Acid Chemical, For Agriculture, 99%View Details
Industrial Grade Erucic Acid Chemical, For Agriculture, 99%View Details
112-86-7 -
 Erucic AcidView Details
Erucic AcidView Details
112-86-7
