Erdosteine
- CAS NO.:84611-23-4
- Empirical Formula: C8H11NO4S2
- Molecular Weight: 249.31
- MDL number: MFCD00867664
- EINECS: 642-172-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
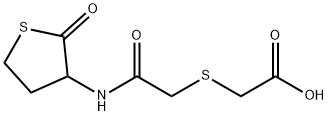
What is Erdosteine?
Description
Erdosteine is a new expectorant introduced in France for the treatment of chronic bronchitis. Erdosteine possesses mucomodulator, mucolytic, mucokinetic and free radical scavenging properties. Its therapeutic efficacy has been demonstrated in patients affected by congestion and viscous mucus in higher and lower respiratory tracts as in acute and chronic bronchitis, sinusitis, otitis, tracheopharyngitis and coryza. Erdosteine acts through its active metabolites containing free sulfhydryl groups which are able to depolymerize the mucopolysaccharides of the excreatum by breaking the disulfide linkages (thereby lowing its viscosity). The prodrug approach provides unique characteristics to erdosteine by offering stable therapeutic efficacy and prevention of the gastric mucus from lythic phenomena. The agent was also reported to have local antiinflammatory and antielastase properties and enhances penetration of antibiotics into bronchial mucus.
Description
Erdosteine is a mucolytic agent and an antioxidant. It inhibits LPS-induced IκB kinase (IKK) activity, NF-κB-mediated transcription, and production of IL-6 and IL-1β in RAW 264.7 cells. Erdosteine (7.5 mg/kg) prevents depletion of renal catalase and glutathione peroxidase (GPX) and increases in renal malondialdehyde (MDA) and nitric oxide (NO) levels in a rat model of nephrotoxicity induced by cisplatin . It reduces spinal cord lipid peroxidation and destruction of spinal motor neurons in a rabbit model of spinal cord ischemia and reperfusion injury induced by thoracoabdominal aortic clamping. Erdosteine (150 mg/kg) reduces lung weight, MDA and protein carbonyl levels, and macrophage and neutrophil accumulation in a mouse model of acute lung injury induced by oleic acid (Item Nos. 90260 | 24659). Formulations containing erdosteine have been used in the treatment of chronic obstructive pulmonary disease (COPD).
Chemical properties
Off-White Solid
Originator
Refarmed (Switzerland)
The Uses of Erdosteine
Erdosteine is a mucolytic. Erdosteine was developed for the treatment of chronic obstructive bronchitis.
The Uses of Erdosteine
A mucolytic, developed for the treatment of chronic obstructive bronchitis.
Indications
Fro the treatment of chronic bronchitis in adults.
Background
Erdosteine is a drug that causes a breakdown of mucus, also known as a mucolytic agent. It is a thiol derivative produced for the clinical management of chronic obstructive bronchitis, in addition to infective exacerbations of chronic bronchitis. This drug contains sulfhydryl groups which are released after erdosteine undergoes hepatic first pass metabolism. Three active metabolites result and possess mucolytic activity in addition to free radical scavenging activity. Erdosteine acts to control mucus production and control its viscosity while increasing mucociliary transport. It also combats the effects of free radicals resulting from cigarette smoke. Erdosteine has been shown to be safe and well tolerated in clinical trials. Erdosteine 300mg twice daily reduced cough (both frequency and severity) and sputum viscosity more quickly and more effectively than placebo and reduced the adhesivity of sputum more effectively than ambroxol 30mg twice daily. Co-administration of erdosteine and amoxicillin in patients with acute infective exacerbation of chronic bronchitis resulted in higher concentrations of the antibiotic in the sputum, leading to earlier and more pronounced amelioration of clinical symptoms compared with placebo. Erdosteine is associated with a low incidence of adverse events, most of which are gastrointestinal and generally mild.
What are the applications of Application
Erdosteine is a homocysteine-derived expectorant
Definition
ChEBI: Erdosteine is a N-acyl-amino acid.
brand name
Edirel; Vectrine
Metabolism
Not Available
Properties of Erdosteine
| Melting point: | 156-160°C |
| Boiling point: | 590.4±50.0 °C(Predicted) |
| Density | 1.48±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | Acetonitrile (Slightly), DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 3.71(at 25℃) |
| color | White to Off-White |
| λmax | 236nm(H2O)(lit.) |
| Merck | 14,3647 |
| CAS DataBase Reference | 84611-23-4(CAS DataBase Reference) |
Safety information for Erdosteine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H320:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Erdosteine
| InChIKey | QGFORSXNKQLDNO-UHFFFAOYSA-N |
Related products of tetrahydrofuran
![9-Chloro-2-methyl-5H-pyrazolo[1,5-d][1,4]benzodiazepin-6(7H)-one](https://img.chemicalbook.in/CAS2/GIF/84661-23-4.gif)


You may like
-
![84611-23-4 2-[2-oxo-2-[(2-oxothiolan-3-yl)amino]ethyl]sulfanylacetic acid (Erdosteine) 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/954a6cd3-ed49-487f-8fb3-b9a3297240b2.png) 84611-23-4 2-[2-oxo-2-[(2-oxothiolan-3-yl)amino]ethyl]sulfanylacetic acid (Erdosteine) 98%View Details
84611-23-4 2-[2-oxo-2-[(2-oxothiolan-3-yl)amino]ethyl]sulfanylacetic acid (Erdosteine) 98%View Details
84611-23-4 -
 84611-23-4 98%View Details
84611-23-4 98%View Details
84611-23-4 -
 Erdosteine 98% CAS 84611-23-4View Details
Erdosteine 98% CAS 84611-23-4View Details
84611-23-4 -
 Erdosteine CAS 84611-23-4View Details
Erdosteine CAS 84611-23-4View Details
84611-23-4 -
 Erdosteine CAS 84611-23-4View Details
Erdosteine CAS 84611-23-4View Details
84611-23-4 -
 106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 Para Chloro Toluene (PCT) 99.00%View Details
106-43-4 -
 Pyrrolidine 99.00%View Details
Pyrrolidine 99.00%View Details
123-75-1 -
 12029-98-0 99.00%View Details
12029-98-0 99.00%View Details
12029-98-0