Aspartame
Synonym(s):Aspartame;Asp-Phe methyl ester;Asp-Phe-OMe;N-(L -α-Aspartyl)-L -phenylalanine methyl ester;Anti-cmer
- CAS NO.:22839-47-0
- Empirical Formula: C14H18N2O5
- Molecular Weight: 294.31
- MDL number: MFCD00002724
- EINECS: 245-261-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-18 17:17:19
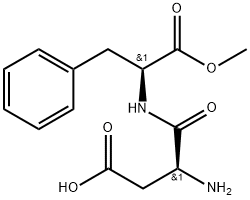
What is Aspartame?
Description
Aspartame is the most popular artificial sweetener in the United States. It is sold as sweeteners such as NutraSweet and Equal, but it is also incorporated into thousands of food products.
Description
The sweetener aspartame is a dipeptide ester sold under the tradenames Equal and NutraSweet, among others. It is formally a condensation product of aspartic acid with the methyl ester of phenylalanine; but the actual synthetic methods are more complex.
In 1968, a South African patent for synthesizing aspartame and other dipeptide sweeteners was awarded to James M. Schlatter and assigned to G. D. Searle (Chicago), now part of Pfizer. The equivalent US Patent (3,492,131) was awarded in 1970. But not until 1981 did the US Food and Drug Administration approve it for use in foods.
At one time, rumors held that aspartame is carcinogenic; but studies performed from 2006 through 2015 refuted this allegation. The FDA and National Cancer Institute supported these studies.
Aspartame’s sweetness is ≈200 times greater than that of sucrose. Its solubility and stability in aqueous media depend strongly on pH. It is most stable at pH 4.3, which makes it ideal for sweetening carbonated beverages. It is unstable at normal cooking and baking temperatures; but it can be used in “no-heat” recipes.
Aspartame is considered to mimic the qualities of sucrose (e.g., flavor, taste duration, and low aftertaste) better than other artificial sweeteners.
Description
Aspartame is a synthetic non-caloric sweetener that is metabolized to phenylalanine, aspartic acid, and methanol in the gut. Aspartame (80 mg/kg per day for 90 days) increases plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activity, induces hepatocyte degeneration and leukocyte infiltration in the liver, and reduces hepatic levels of reduced glutathione (GSH), oxidized glutathione (GSSG), and γ-glutamylcysteine (γ-GC) in mice. Formulations containing aspartame have been used as sweetening agents and flavor enhancers in foods and beverages.
Chemical properties
white powder or tablets
Chemical properties
Aspartame (N-L-aspartyl-L-phenylalanine-1-methyl ester, 3-amino-N-(a-carbomethoxy-
phenethyl)-succinamic acid-N-methyl ester) is an intense sweetener widely
used in foods and beverages. Its solubility in water is approximately 10 g/L at room
temperature. Aspartame is not fully stable under common processing and storage
conditions of foods and beverages with the highest stability around pH 4.3.
Aspartame is about 200 times sweeter than sucrose with a clean, but slightly
lingering sweetness. It is used as the single sweetener, but often also in blends with other intense sweeteners owing to synergistic taste enhancement and taste quality
improvement often seen in such blends.
In the European Union, aspartame is approved as E 951 for a large number of
food applications. In the United States, it is approved as a multipurpose sweetener
for food and beverage uses and it is also approved in many other countries.
Chemical properties
Aspartame occurs as an off white, almost odorless crystalline powder with an intensely sweet taste.
Chemical properties
Aspartame has no odor, but has an intense sweet taste. It is a high intensity sweetener, about 160 to 200 times sweeter than sucrose. Normal digestive processes convert aspartame to phenylalanine, aspartic acid and methanol. Metabolism of aspartame in the body provides approximately 17 kJ (4 kcal)/g. The stability of aspartame is affected by moisture, pH and temperature. For a detailed description of this compound, refer to Burdock (1997).
Originator
Canderel,Searle,France,1979
History
Aspartame was discovered accidentally in 1965 during a search for drugs to treat gastric ulcers. James M. Schlatter, an organic chemist working for G. D. Searle & Company, was using aspartyl-phenylalanine methyl ester (aspartame) in a synthesis procedure and inadvertently got some of the compound on his hands.
The Uses of Aspartame
Aspartame is a high-intensity sweetener that is a dipeptide, provid- ing 4 cal/g. it is synthesized by combining the methyl ester of phenylalanine with aspartic acid, forming the compound n-l-alpha- aspartyl-l-phenylalanine-1-methyl ester. it is approximately 200 times as sweet as sucrose and tastes similar to sugar. it is compara- tively sweeter at low usage levels and at room temperature. its mini- mum solubility is at ph 5.2, its isoelectric point. its maximum solubility is at ph 2.2. it has a solubility of 1% in water at 25°c. the solubility increases with temperature. aspartame has a certain insta- bility in liquid systems which results in a decrease in sweetness. it decomposes to aspartylphenylalanine or to diketropiperazine (dkp) and neither of these forms is sweet. the stability of aspartame is a function of time, temperature, ph, and water activity. maximum stability is at approximately ph 4.3. it is not usually used in baked goods because it breaks down at the high baking temperatures. it contains phenylalanine, which restricts its use for those afflicted with phenylketonuria, the inability to metabolize phenylalanine. uses include cold breakfast cereals, desserts, topping mixes, chew- ing gum, beverages, and frozen desserts. the usage level ranges from 0.01 to 0.02%.
The Uses of Aspartame
A dipeptide ester about 160 times sweeter than sucrose in aqueous solution. A non-nutritive sweetener.
The Uses of Aspartame
Non-nutritive sweetener.
The Uses of Aspartame
Aspartame in powder form for limited uses such as cereals, powdered drinks, and chewing gum. When aspartame is used in baked goods and baking mixes, it should not exceed 0.5% by weight. Packages of the dry, free-fl owing aspartame are required to prominently display the sweetening equivalence in teaspoons of sugar.
The Uses of Aspartame
The chemical name for aspartame is L-aspartyl-L-phenylalamine methyl
ester.
It is a white crystalline powder and is about 200 times as sweet as
sucrose. It is noted for a clean, sweet taste that is similar to that of
sucrose.
Aspartame is the most widely used artificial sweetener in the world.
It was approved by the FDA for use in the USA in 1981, and now is
approved for use in several other countries of the world. One of the
drawbacks of aspartame is its instability to heat and acid. Under acidic
conditions aspartame slowly hydrolyzes leading to a loss of sweetness,
chemical interaction, and microbial degradation. The shelf life of the
aspartame-sweetened products with high water content is limited to
about 6 months, after which it breaks down into its constituent components
and loses its sweetening abilities. At elevated temperatures, solid
aspartame slowly releases methanol to form aspartyl phenylalamine
and the dioxopiperazine. This reaction is especially favored at neutral and
alkaline pH values. Because of this reason, aspartame cannot be used
in hot baking foods.
Another disadvantage of aspartame was noticed in the human digestive
system. When the body ingests aspartame, it breaks down into its
three constituent components: phenylalamine, aspartate, and methanol.
The phenylalamine and aspartate are handled by enzymes in the stomach
and in the small intestine, while the methanol is transported to the
liver for detoxification. The metabolism of phenylalamine requires an
enzyme that is not produced by a small proportion of the population
having a genetic disorder called phenyl keton uria (PKU). Aspartame
should be avoided by persons suffering from PKU. A warning to PKU sufferers
on aspartame-containing products is required in many countries.
Definition
ChEBI: A dipeptide composed of methyl L-phenylalaninate and L-aspartic acid joined by a peptide linkage.
Production Methods
Aspartame is produced by coupling together L-phenylalanine (or Lphenylalanine methyl ester) and L-aspartic acid, either chemically or enzymatically. The former procedure yields both the sweet aaspartame and nonsweet β-aspartame from which the α-aspartame has to be separated and purified. The enzymatic process yields only α-aspartame.
Production Methods
Aspartame is synthesized using the L enantiomer of phenylalanine. The L enantiomer is separated from the D enantiomer, the racemic mixture, by reacting it with acetic anhydride (CH32
By coupling the amino acids L-phenylalanine and L-aspartic acid, and the esterification of the carboxyl group of the
phenylalanine moiety to produce the methyl ester. This esterification can occur before or after coupling. The crystallized slurry is
centrifuged and the resulting “wet-cake” is washed to remove impurities. A solution of 88.5 parts of L-phenylalanine methyl ester hydrochloride in 100
parts of water is neutralized by the addition of dilute aqueous potassium
bicarbonate, then is extracted with approximately 900 parts of ethyl acetate.
The resulting organic solution is washed with water and dried over anhydrous
magnesium sulfate. To that solution is then added 200 parts of Nbenzyloxycarbonyl-
L-aspartic acid α-p-nitrophenyl, β-benzyl diester, and that
reaction mixture is kept at room temperature for about 24 hours, then at
approximately 65°C for about 24 hours. The reaction mixture is cooled to
room temperature, diluted with approximately 390 parts of cyclohexane, then
cooled to approximately -18°C in order to complete crystallization. The
resulting crystalline product is isolated by filtration and dried to afford β-
benzyl N-benzyloxycarbonyl-L-aspartyl-L-phenylalanine methyl ester, melting
at about 118.5-119.5°C. Sugar supplement Aspartame is produced from L-aspartic acid and L-phenylalanine and methanol or
alternatively L-phenylalanine methyl ester. The standard process uses common
chemical methods of peptide synthesis. Enzymatic coupling of the two amino
acids is also possible. N-formyl-L-aspartic acid and L- or D.L-phenylalanine methyl
ester can be condensed to aspartame by thermolysin-like proteases. The
formylated aspartame can be deformylated chemically or with a formylmethionyl
peptide deformylase to yield the sweetener.The enzymatic coupling does not
require L-phenylalanine but can start from the racemic product obtained in
chemical synthesis, and the remaining D-phenylalanine can be racemized again. Asp-Phe methyl ester (aspartame, APM, ASP), a dipeptide ester, is made up of phenyl alanine and aspartic acid. Its genotoxic effects have been investigated. Its interaction with certain hydrocolloids has been studied. Aspartame is used as an intense sweetening agent in beverage
products, food products, and table-top sweeteners, and in
pharmaceutical preparations including tablets, powder mixes,
and vitamin preparations. It enhances flavor systems and can be
used to mask some unpleasant taste characteristics; the approximate
sweetening power is 180–200 times that of sucrose. Asp-Phe methyl ester (Asp-Phe-OMe) is used as a synthetic sweeter, sugar substitute. Asp-Phe methyl ester is being studied for a variety of potential benefits as a nutrition supplement, such as the delay of osteoarthritis and modulation of rheumatoid factor activity. Asp-Phe methyl ester is being studied for its effect on thrombin activity and blood clotting. Human systemic effects byingestion: allergic dermatitis. Experimental reproductiveeffects. When heated to decomposition it emits toxicfumes of NOx. Aspartame is widely used in oral pharmaceutical formulations,
beverages, and food products as an intense sweetener, and is
generally regarded as a nontoxic material. However, the use of
aspartame has been of some concern owing to the formation of the
potentially toxic metabolites methanol, aspartic acid, and phenylalanine.
Of these materials, only phenylalanine is produced in
sufficient quantities, at normal aspartame intake levels, to cause
concern. In the normal healthy individual any phenylalanine
produced is harmless; however, it is recommended that aspartame
be avoided or its intake restricted by those persons with
phenylketonuria. Aspartame is nontoxic. However, individuals with the rare,
genetic disease, phenylketonuria (PKU), cannot properly
metabolize phenylalanine. Such individuals are detected by
testing at birth and placed on special low-phenylalanine diets
to control their blood phenylalanine concentrations. Thus,
PKU individuals need to be aware that aspartame is a source of
phenylalanine. The rate of aspartame degradation is faster in a
phosphate buffer solution than in a citrate buffer
solution at the same pH and buffer concentration. The
primary mechanism by which aspartame degrades, the
formation of diketo piperazine, involves the
nucleophilic attack of carbonyl by the free amine,
which requires proton transfer. Aspartame is stable in dry conditions. In the presence of moisture,
hydrolysis occurs to form the degradation products L -aspartyl-Lphenylalanine
and 3-benzyl-6-carboxymethyl-2,5-diketopiperazine
with a resulting loss of sweetness. A third-degradation product is
also known, β-L-aspartyl-L-phenylalanine methyl ester. For the
stability profile at 258℃ in aqueous buffers. Differential scanning calorimetry experiments with some directly
compressible tablet excipients suggests that aspartame is incompatible
with dibasic calcium phosphate and also with the lubricant
magnesium stearate. Reactions between aspartame and sugar
alcohols are also known. Accepted for use as a food additive in Europe and in the USA.
Included in the FDA Inactive Ingredients Database (oral powder for
reconstitution, buccal patch, granules, syrups, and tablets).
Included in nonparenteral medicines licensed in the UK. Included
in the Canadian List of Acceptable Non-medicinal Ingredients.
Preparation
Manufacturing Process
To a solution of 180 parts of β-benzyl N-benzyloxycarbonyl-L-aspartyl-Lphenylalanine
methyl ester in 3,000 parts by volume of 75% acetic acid is
added 18 parts of palladium black metal catalyst, and the resulting mixture is
shaken with hydrogen at atmospheric pressure and room temperature for
about 12 hours. The catalyst is removed by filtration, and the solvent is
distilled under reduced pressure to afford a solid residue, which is purified by
recrystallization from aqueous ethanol to yield L-aspartyl-L-phenylalanine
methyl ester. It displays a double melting point at about 190°C and 245-
247°C.
Therapeutic Function
Biotechnological Production
Production processes based on fermentation are available for the two main
components, aspartic acid and phenylalanine.
General Description
Pharmaceutical Applications
Unlike some other intense sweeteners, aspartame is metabolized
in the body and consequently has some nutritive value: 1 g provides
approximately 17 kJ (4 kcal). However, in practice, the small
quantity of aspartame consumed provides a minimal nutritive
effect.
Biochem/physiol Actions
Safety Profile
Safety
The WHO has set an acceptable daily intake for aspartame at up
to 40 mg/kg body-weight. Additionally, the acceptable daily
intake of diketopiperazine (an impurity found in aspartame) has
been set by the WHO at up to 7.5 mg/kg body-weight.
A number of adverse effects have been reported following the
consumption of aspartame, particularly in individuals who
drink large quantities (up to 8 liters per day in one case) of
aspartame-sweetened beverages. Reported adverse effects include:
headaches; grand mal seizure;memory loss;gastrointestinal
symptoms; and dermatological symptoms. However, scientifically
controlled peer-reviewed studies have consistently failed to
produce evidence of a causal effect between aspartame consumption
and adverse health events. Controlled and thorough studies
have confirmed aspartame’s safety and found no credible link
between consumption of aspartame at levels found in the human
diet and conditions related to the nervous system and behavior, nor
any other symptom or illness. Aspartame is well documented to be
nongenotoxic and there is no credible evidence that aspartame is
carcinogenic.
Although aspartame has been reported to cause hyperactivity
and behavioral problems in children, a double-blind controlled trial
of 48 preschool-age children fed diets containing a daily intake of
38 ± 13 mg/kg body-weight of aspartame for 3 weeks showed no
adverse effects attributable to aspartame, or dietary sucrose, on
children’s behavior or cognitive function.
Environmental Fate
Metabolic pathway
Storage
Stability in aqueous solutions has been enhanced by the addition
of cyclodextrins, and by the addition of polyethylene glycol 400
at pH 2. However, at pH 3.5–4.5 stability is not enhanced by the
replacement of water with organic solvents.
Aspartame degradation also occurs during prolonged heat
treatment; losses of aspartame may be minimized by using processes
that employ high temperatures for a short time followed by rapid
cooling.
The bulk material should be stored in a well-closed container, in
a cool, dry place.
Incompatibilities
Regulatory Status
Properties of Aspartame
| Melting point: | 242-248 °C |
| Boiling point: | 436.08°C (rough estimate) |
| alpha | 15.5 º (c=4, 15N formic acid) |
| Density | 1.2051 (rough estimate) |
| refractive index | 14.5 ° (C=4, 15mol/L Formic Acid) |
| storage temp. | 2-8°C |
| solubility | Sparingly soluble or slightly soluble in water and in ethanol (96 per cent), practically insoluble in hexane and in methylene chloride. |
| form | Powder |
| appearance | white crystalline powder or colorless needles |
| pka | pKa 3.19±0.01 (H2O t=25.0 I=0.100(NaCl))(Approximate);7.87±0.02(H2O t=25.0 I=0.100(NaCl))(Approximate) |
| color | White |
| Odor | odorless with a sweet taste |
| PH | pH(8g/l, 25℃) : 4.5~6.0 |
| Water Solubility | Soluble in formic acid, dimethyl sulfoxide. Sparingly soluble in water and ethanol. |
| Merck | 14,839 |
| BRN | 2223850 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 22839-47-0(CAS DataBase Reference) |
| EPA Substance Registry System | L-Phenylalanine, L-.alpha.-aspartyl-, 2-methyl ester (22839-47-0) |
Safety information for Aspartame
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aspartame
| InChIKey | IAOZJIPTCAWIRG-QWRGUYRKSA-N |
Aspartame manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Aspartame 98%View Details
Aspartame 98%View Details -
 Aspartame 99%View Details
Aspartame 99%View Details -
 Aspartame >98% (HPLC) CAS 22839-47-0View Details
Aspartame >98% (HPLC) CAS 22839-47-0View Details
22839-47-0 -
 Aspartame 97% CAS 22839-47-0View Details
Aspartame 97% CAS 22839-47-0View Details
22839-47-0 -
 Aspartame 98% CAS 22839-47-0View Details
Aspartame 98% CAS 22839-47-0View Details
22839-47-0 -
 Aspartame 95% CAS 22839-47-0View Details
Aspartame 95% CAS 22839-47-0View Details
22839-47-0 -
 Aspartame Artificial Sweetener Powder, Packaging Size: 25 KgView Details
Aspartame Artificial Sweetener Powder, Packaging Size: 25 KgView Details
22839-47-0 -
 AspartameView Details
AspartameView Details
22839-47-0
