Enzastaurin (LY317615)
Synonym(s):3-(1-Methyl-1H-indol-3-yl)-4-[1-[1-(2-pyridinylmethyl)-4-piperidinyl]-1H-indol-3-yl]-1H-pyrrole-2,5-dione;D04014;LY317615;LY-317615
- CAS NO.:170364-57-5
- Empirical Formula: C32H29N5O2
- Molecular Weight: 515.6
- MDL number: MFCD11040980
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
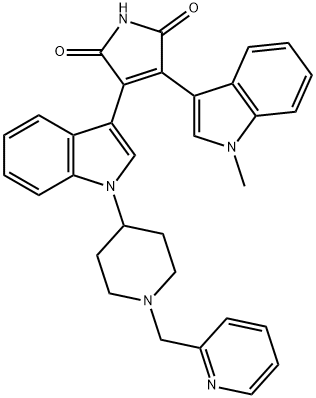
What is Enzastaurin (LY317615)?
Description
Enzastaurin (170364-57-5) is a potent and selective PKCβ inhibitor.? IC50 = 6, 39, 83 and 110 nM, for PKCβ, PKCα, PKCγ and PKCε respectively.1 Induces apoptosis in multiple myeloma cell lines via inhibition of the AKT signaling pathway.2 Induces mitotic missegregation and preferential cytotoxicity in colorectal cancer cells with chromosomal instability.3 Attenuates amphetamine-stimulated dopamine efflux.4 Inhibits blood-brain barrier leakiness in a mouse model.5
The Uses of Enzastaurin (LY317615)
PKCβ-selective inhibitor, suppresses angiogenesis.
The Uses of Enzastaurin (LY317615)
Enzastaurin has been used in splicing analysis to study its effects on splicing of a mutated exon.
What are the applications of Application
Enzastaurin is a potent inhibitor of PKCβ, PKCα, PKCγ and PKCε
Definition
ChEBI: 3-(1-methyl-3-indolyl)-4-[1-[1-(2-pyridinylmethyl)-4-piperidinyl]-3-indolyl]pyrrole-2,5-dione is a member of indoles and a member of maleimides.
Biochem/physiol Actions
Enzastaurin is a potent and PKCβ preferring inhibitor. Also, Enzastaurin inhibits AKT and GSK3β. Enzastaurin acts as anti-angiogenic and antineoplastic agent.
References
1) Graff et al. (2005), The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts; Cancer Res. 65 7462 2) Rizvi et al. (2006) Enzastaurin (LY317615), a protein kinase Cβ inhibitor, inhibits the AKT pathway and induces apoptosis in multiple myeloma cell lines; Mol.Cancer Ther. 5 1783 3) Ouaret and Larsen (2014), Protein kinase Cβ inhibition by enzastaurin leads to mitotic missegregation and preferential cytotoxicity toward colorectal cancer cells with chromosomal instability (CIN); Cell Cycle 13 2697 4) Zestos et al. (2016), PKCβ Inhibitors Attenuate Amphetamine-Stimulated Dopamine Efflux; ACS Chem.Neurosci. 7 757 5) Stranahan et al. (2016), Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice; J.Cereb.Blood Flow Metab. 36 2108
Properties of Enzastaurin (LY317615)
| Melting point: | 249-261℃ |
| Boiling point: | 767.2±60.0 °C(Predicted) |
| Density | 1.34 |
| RTECS | UX9626850 |
| Flash point: | 417.8℃ |
| storage temp. | -20°C |
| solubility | DMSO: soluble10mg/mL, clear (warmed) |
| form | powder |
| pka | 7.88±0.60(Predicted) |
| color | , light orange to dark orange-red |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20°C for up to 3 months. |
Safety information for Enzastaurin (LY317615)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Enzastaurin (LY317615)
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 Enzastaurin 98% (HPLC) CAS 170364-57-5View Details
Enzastaurin 98% (HPLC) CAS 170364-57-5View Details
170364-57-5 -
 Enzastaurin CAS 170364-57-5View Details
Enzastaurin CAS 170364-57-5View Details
170364-57-5 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4