Emamectin benzoate
- CAS NO.:137512-74-4
- Empirical Formula: C49H77NO13
- Molecular Weight: 888.13
- MDL number: MFCD32206651
- EINECS: 415-130-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-13 11:39:26
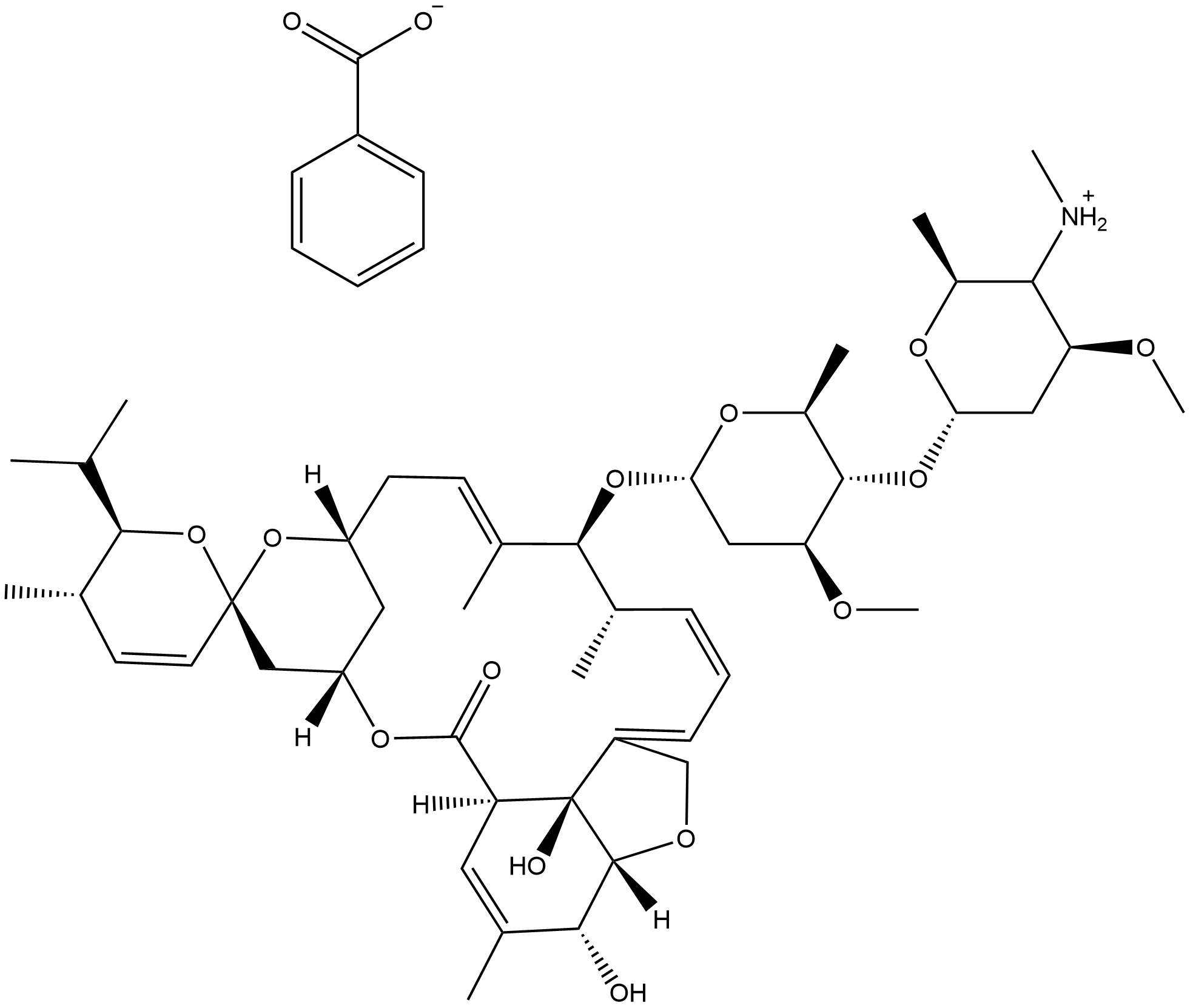
What is Emamectin benzoate?
Chemical properties
White Crystalline Powder
The Uses of Emamectin benzoate
Emamectin benzoate is a second-generation avermectin insecticide highly effective against a broad range of lepidopteran larvae and certain other insects.
The Uses of Emamectin benzoate
A mixture of semi-synthetic Avermectins. Exists as the anhydrous and various hydrated forms having different crystal morphologies. Insecticide
The Uses of Emamectin benzoate
Insecticide.
What are the applications of Application
Emamectin B1 Benzoate is a neurotoxic pesticidal compound
Metabolic pathway
In laboratory studies, emamectin benzoate is relatively stable to aqueous hydrolysis (acid, neutral and alkaline) under dark conditions. Emamectin benzoate degrades extensively in soil via microbial action to multiple degradation products including the 8a-oxidation and 8a-hydroxylation products. CO2 and soil-bound residues were the major terminal residues which are incorporated into humic and fulvic acid fractions. In plants, emamectin benzoate degrades extensively to multiple polar components including the N-demethylated, N-formylated and conjugated products. Probably due to rapid elimination in faeces, the metabolic transformation of emamectin benzoate in animals is minimal. However, N-demethylation was observed as the major metabolic pathway. The hydrolytic, photolytic and overall metabolic pathways of emamectin benzoate in soils, plants and animals are presented in Schemes 1,2 and 3.
Degradation
Emamectin benzoate (1) was relatively stable to hydrolysis in acidic, neutral
and alkaline solutions (pH 5, 7 and 9) at 25 °C, with less than 10%
degradation occurring after 30 days. It hydrolysed most rapidly in acidic
solution (pH 5) at 25 °C with a DT50 of 136 days (Chukwudebe, 1992). Two
polar components (each <10%) eluted close to the void volume under
reversed phase chromatographic conditions.
The photodegradation half-lives of emamectin benzoate (10-12 mg 1-1)
in buffered distilled water (pH 7) containing 1% (v/v) acetonitrile, ethanol
or acetone as cosolvent under continuous exposure to a xenon lamp
were 65,9 and 0.5 days, respectively. In both buffered and natural pond
water, the major photodegradation products included 8,9-MAB1a (2), 8a-hydroxylated
MAB1a (3) and minor unknown polar residues (Scheme 1).
In sensitised buffered water, 8a-oxo-MAB1a (4) and 10,11-14,15-MAB1a
diepoxide (5) were found as additional residual products (Mushtaq et al.,
1997).
Properties of Emamectin benzoate
| Melting point: | 146-150°C |
| alpha | D -6.9° (c = 0.5% in methanol) |
| vapor pressure | 4 x 10-7mPa |
| storage temp. | Refrigerator |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| Water Solubility | 320 mg l-1 (pH 5), 24 mg l-1 (pH 7),
0.1 mg l-1 (pH 9) |
| pka | 4.2; 7.6(at 25℃) |
| form | Solid |
| color | Pale Yellow to Light Yellow |
Safety information for Emamectin benzoate
Computed Descriptors for Emamectin benzoate
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4