(E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
- CAS NO.:886230-77-9
- Empirical Formula: C19H18IN3O
- Molecular Weight: 431.27
- MDL number: MFCD12405576
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-19 09:58:18
![(E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole Structural](https://img.chemicalbook.in/CAS2/GIF/886230-77-9.gif)
What is (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole?
The Uses of (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
(E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole is an intermediate used to prepare Axitinib (A794650) as a tyrosine kinase inhibitor. Axitinib is used in cancer therapy.
Synthesis
6-amino-3-((E)-2-pyridin-2-yl-vinyl)-1-(tetrahydropyran-2-yl)-1 H-indazole (100 g) dissolved in acetic acid (6.5 L) is added over 1.5 hours to a solution of sodium nitrite (35 g) dissolved in water (3.0 L) at 0℃ (-3 ± 3°C). The mixture is stirred for 1 hour at 0℃, and a solution of hydrochloric acid (560 mL diluted in 1 L of water) at 0℃ is added over 15 minutes. The mixture is stirred for 1 hour at 0℃. The formation of the diazonium salt is monitored by HPLC. Dicholoromethane (400 ml) at O0C is added over 10 minutes to the diazonium salt solution at 0℃, and a solution of potassium iodide (207. 25 g) dissolved in water (300 ml) at O0C is added over 1.5 hours. The reaction mixture is agitated for 3 hours at 0℃ (until complete by HPLC). The mixture is then poured into a solution of sodium bisulfite in process water [Sodium bisulfite (200g) dissolve in process water (500mL) at 27± 3°C] and Dicholoromethane (400 ml) below 270C, agitated, and the layers separated. The aqueous layer is extracted with Dichloromethane (100 ml) at 27℃ and combined. A solution of aqueous ammonia (100 ml) at 27± 3°C is added over 40 minutes to the combined organic layers until the aqueous phase is basic (pH = 9 to 12). Distill out dichloromethane and add methanol and heat to 50 ± 3°C and stir it at this temperature for 15 min and then stir for 30 min at RT, followed by washing with methanol. Add dichloromethane and heat to 45± 3°C and add activated carbon at this temperature. Followed by addition of methanol and dichloromethane (if required) and stir the reaction mixture at 27± 3°C for 30 min and cool it to 0±3°C and stir 1 hr and wash with methanol to provide (E)-6-Iodo-3-[2- (pyridine-2yl)ethenyl]-1 -(tetrahydro-2H- pyran-2-yl)-1H-indazole.
References
[1] LESLIEISLA. Avelumab and axitinib in the treatment of renal cell carcinoma: safety and efficacy.[J]. Expert Review of Anticancer Therapy, 2020, 20 5: 343-354. DOI:10.1080/14737140.2020.1756780.
Properties of (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
| Melting point: | 156-159°C |
| Boiling point: | 561.2±50.0 °C(Predicted) |
| Density | 1.60 |
| storage temp. | 2-8°C(protect from light) |
| solubility | Chloroform, DMSO, Methanol |
| form | Solid |
| pka | 4.25±0.10(Predicted) |
| color | Light Yellow |
| Stability: | Hygroscopic, Light Sensitive |
Safety information for (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
Computed Descriptors for (E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
(E)-6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole manufacturer
Rivashaa Agrotech Biopharma Pvt. Ltd.
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
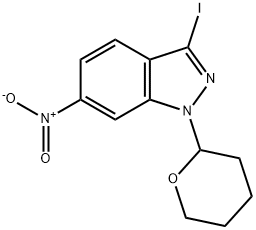
![(E)-6-Nitro-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole](https://img.chemicalbook.in/CAS2/GIF/886230-75-7.gif)

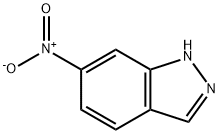
![(E)-3-[2-(Pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazol-6-amine](https://img.chemicalbook.in/CAS2/GIF/886230-76-8.gif)
You may like
-
![886230-77-9 (E)-6-Iodo-3-[2-(Pyridin-2-Yl) Ethenyl]-1-(Tetrahydro-2H-Pyran- 2-Yl)-1H-Indazole 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/dd3616ad-97d6-4e2d-94af-9b3880a8152b.png) 886230-77-9 (E)-6-Iodo-3-[2-(Pyridin-2-Yl) Ethenyl]-1-(Tetrahydro-2H-Pyran- 2-Yl)-1H-Indazole 99%View Details
886230-77-9 (E)-6-Iodo-3-[2-(Pyridin-2-Yl) Ethenyl]-1-(Tetrahydro-2H-Pyran- 2-Yl)-1H-Indazole 99%View Details
886230-77-9 -
 886230-77-9 99%View Details
886230-77-9 99%View Details
886230-77-9 -
![6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/0132842d-7df6-4c29-a456-cbb033e24e99.png) 6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 98%View Details
6-Iodo-3-[2-(pyridin-2-yl)ethenyl]-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole 98%View Details
886230-77-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1