DOXEPIN
- CAS NO.:1668-19-5
- Empirical Formula: C19H21NO
- Molecular Weight: 279.38
- MDL number: MFCD00865448
- EINECS: 214-966-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-10-28 16:00:23
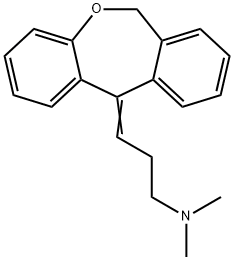
What is DOXEPIN?
Absorption
Doxepin is moderately absorbed following oral ingestion with a bioavailability of 30%. The median peak concentration of doxepin ranges from 8.8-45.8 ng/ml and it is achieved 3.5 hours after initial administration. Its absorption is increased with concomitant administration of a high-fat meal.
Toxicity
Oral LD50 values of doxepin in mouse and rat are 180 mg/kg and 147 mg/kg, respectively. In an overdose state, symptoms of convulsions, dysrhythmias, coma, severe hypotension, central nervous system depression, changes on electrocardiography results and death have been observed.
On fertility studies, doxepin was shown to increase the copulatory interval, decrease the corpora lutea, decrease implantation, decreased the number of viable embryos, decrease litter size, increase the number of abnormal sperm and decrease the sperm motility. There is no evidence indicating carcinogenic and mutagenic potential.
Originator
Sinequan,Pfizer,US,1969
The Uses of DOXEPIN
Used clinically to treat anxiety and depression. Antidepressant
Background
Doxepin is a psychotropic agent with antidepressant and anxiolytic properties. It is a tertiary amine that can be presented as (E) and (Z) stereoisomers with the (Z) stereoisomer corresponding to cidoxepin. Doxepin commonly produces a 5:1 (E):(Z) racemic mixture.
In a strict sense, doxepin is not a tricyclic antidepressant but it is commonly associated with the class since it shares a lot of properties with members of the drug family including amitriptyline, clomipramine, desipramine, imipramine, nortriptyline, protriptyline and trimipramine.
Doxepin was developed by Pfizer and FDA approved in 1969 as an antidepressant. However, in 2010 it was approved for the treatment of insomnia. The latter indication was presented by Pernix Therapeutics.
Indications
Oral doxepin is approved for the following indications:
Topical doxepin is also approved for short-term (up to 8 days) management of moderate pruritus in adult patients with atopic dermatitis, pruritus or lichen simplex chronicus.
Off-label, doxepin is used topically for the management of neuropathic pain.
Depression is a common medical illness that causes feelings of sadness and or loss of interest in prior enjoyable activities. This condition can lead to emotional and physical disturbances that can decrease the ability of a person to function in a regular environment.
Anxiety is a normal reaction of the body towards a normal danger. When the anxious state is exacerbated or appears on situations without danger, it is defined as an anxiety disorder. This disorders can appear in different forms such as phobias, panic, obsessive-compulsive disorder and post-traumatic stress disorder.
Insomnia is a sleep disorder that directly affects the quality of life of the individual. It is characterized by the complication either to fall asleep or to stay asleep. This condition can be occasional or chronic.
Pruritus is defined as an unpleasant skin reaction that provokes the urge to scratch. It can be localized or generalized and it can appear in an acute or chronic manner.
Neuropathic pain occurs due to the damage or dysfunction of the peripheral or central nervous system rather than stimulation of the pain receptors.
Definition
ChEBI: A dibenzooxepine that is 6,11-dihydrodibenzo[b,e]oxepine substituted by a 3-(dimethylamino)propylidene group at position 11. It is used as an antidepressant drug.
Manufacturing Process
(A) Preparation of 3-bromopropyltriphenylphosphonium bromide:
Triphenylphosphine, 1.0 kg, and 770 grams of 1,3-dibromopropane are
dissolved in 2.0 liters of xylene and the solution is stirred under a nitrogen
atmosphere at 130°C. After 20 hours the mixture is cooled, and the crystalline
product, which precipitates, is collected and washed with 20 liters of benzene.
After drying in vacuo the product weighs 1,578 grams, MP 229°-230°C;titration for bromide ion: Found, 17.1%; calculated, 17.2%.
(B) Preparation of 3-dimethylaminopropyltriphenylphosphonium bromide
hydrobromide: A solution of 595 grams of anhydrous dimethylamine and
1,358 grams of 3-bromopropyl-triphenylphosphonium bromide in 4 liters of
ethanol is warmed to 70°C until solution is complete and the solution then is
allowed to stand at room temperature for 20 hours. Volatile components are
removed by distillation in a vacuum and the residue is suspended in 2.0 liters
of ethanol and is redistilled to remove excess amine. The residue is dissolved
in 3.0 liters of warm ethanol and gaseous hydrogen bromide is passed into
the solution until the mixture is acidic. After filtration the solution is
concentrated to a volume of 3.0 liters, is cooled, whereupon the product
precipitates, and the precipitate is collected; it weighs 1,265 grams, MP 274-
281°C. Recrystallization from ethanol raises the MP to 280.5°-282.5°C.
Bromide ion titration: Found, 31.2%; calculated 31.3%.
(C) Preparation of doxepin: 1,530 grams of the product from step (B) is
suspended in 4.5 liters dry tetrahydrofuran and 6.0 mols of butyl lithium in
heptane is added during 1 hour. After an additional 30 minutes, 483 grams of
6,11-dihydrodibenz-(b,e)oxepin-11-one, prepared as described in Belgian
Patent 641,498, is added to the deep red solution and the reaction was
maintained at reflux for 10 hours. Water, 500 ml, is added at room
temperature and the solvent is removed in vacuo. The crude residue is treated
with 10% hydrochloric acid until acidic (pH 2) and then 1.5 liters benzene is
added. After stirring, the mixture separates into 3 phases (an insoluble
hydrochloride salt product phase, an aqueous phase and an organic phase).
The benzene layer is removed by decantation and the remaining mixture is
rendered basic with 10% sodium hydroxide solution and is extracted with
three 1,500 ml portions of benzene. The benzene extracts are washed, then
dried with anhydrous sodium sulfate and concentrated in a vacuum leaving a
residue of 1,530 grams, gas and thin layer chromatography analysis show this
to be a cis/trans mixture (approx. 4:l) of 11-dimethylaminopropylidene-6,11-
dihydrodibenz-(b,e)oxepin (90% yield). This mixture has substantially more
activity pharmacologically than the cis/trans mixture obtained by the Grignard
route disclosed in the Belgian Patent 641,498. This base is then converted to
the hydrochloride with HCl.
brand name
Apo-doxepin;Co dox;Deptran;Doksapan;Dolat;Doxal;Doxedyn;Doxepin hcl;Gilex;Novo-doxepin;Novoxapin;Sinequan;Sinquan concentrate;Sinquane;Tolllluan;Triadapin;Zonalon.
Therapeutic Function
Tranquilizer
World Health Organization (WHO)
Doxepin, a tricyclic antidepressant was introduced in 1964 for the management of endogenous depression. Much of the adverse effects are caused by its antimuscarinic actions. These include dry mouth, cardiac arrhythmias, central nervous system disturbances, blood disorders and risk of suicide. The risk of suicide and dangers related to overdosage led the Norwegian Medicines Control Authority to put the higher strength formulation under prescribing restriction in 1992. The risk of death following overdosage is apparently higher for products containing tricyclic compounds as compared with nontricyclic products.
Biological Activity
Highly potent H 1 histamine receptor antagonist (K d = 310 pM) and tricyclic antidepressant. Also binds to the H 4 histamine receptor (pK i = 6.79).
Contact allergens
This benzoxepin tricylcic drug has antidepressant, anticholinergic, antiitching, and antihistamine properties. After oral use, it has been developed as a topical antiitching agent. Allergic contact dermatitis is not infrequent.
Pharmacokinetics
Similar to other tricyclic antidepressants, doxepin was shown, in preclinical trials, to decrease the electrical activity of the brain, prolong the hexobarbital-induced sleep and block avoidance behavior without affecting the conditioned emotional response. At high doses, it also produces symptoms of central nervous system depression.
Doxepin is known to cause antidepressant, sedative, and anticholinergic effects. At high doses, its anticholinergic and antiadrenergic properties are the most prevalent which limit its efficacy. These effects are observed at high doses where its affinity for H1 histamine receptor is lost and its binding to other receptors is observed.
The maximal antidepressive effects of doxepin are present around two weeks following initiation of therapy. However, the sedative effects of doxepin, usually used for the treatment of insomnia or anxiety, are observed immediately after administration.
Metabolism
Doxepin is extensively metabolized to N-desmethyldoxepin which is a biologically active metabolite and other inactive metabolites. The first-pass metabolism accounts for 55-87% of the administered dose. After, the secondary metabolism is driven by the transformation of N-desmethyldoxepin to its glucuronide conjugates.
The main metabolic enzymes involved in the transformation of doxepin are the members of the cytochrome P450 family, CYP2C19 and CYP2D6 with minor involvement of CYP1A2 and CYP2C9.
Properties of DOXEPIN
| Melting point: | 187-189°C |
| Boiling point: | bp0.03 154-157°; bp0.2 260-270° |
| Density | 1.0594 (rough estimate) |
| refractive index | 1.5000 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| pka | 9.40±0.28(Predicted) |
| Water Solubility | 31.57mg/L(25 ºC) |
| EPA Substance Registry System | Doxepin (1668-19-5) |
Safety information for DOXEPIN
Computed Descriptors for DOXEPIN
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

![DOXEPIN HYDROCHLORIDE [N-METHYL-3H]](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB2227087.gif)
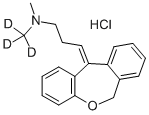
You may like
-
 1668-19-5 98%View Details
1668-19-5 98%View Details
1668-19-5 -
 Doxepin 95% CAS 1668-19-5View Details
Doxepin 95% CAS 1668-19-5View Details
1668-19-5 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
